The Histopathology of Vulvar Neoplasia
Authors
INTRODUCTION
The vulva contains both keratinizing, hair-bearing skin; and non-keratinizing squamous mucosa, as well as subcutaneous soft tissues. Neoplastic disease of the vulva most commonly involves the epidermal layer or squamous mucosal lining, and most of these neoplasms are of squamous origin. As elsewhere on the epidermis and other squamous mucosal linings of the body, the lesions may present as intraepithelial, non-invasive lesions in the early stages, and, if left untreated, may progress to frankly invasive tumors. Less commonly, neoplastic lesions may be encountered in the subcutaneous glands or soft tissue with no involvement of the surface epithelium.
SQUAMOUS INTRAEPITHELIAL LESIONS OF VULVA
The classification schemes and terminologies for intraepithelial neoplasia have evolved significantly over the past several decades. For many years, the vulvar intraepithelial neoplasia (VIN) nomenclature, introduced in the 1980s by the International Society for the Study of Vulvovaginal Disease (ISSVD), in which lesions were stratified into three tiers of severity was the dominant classification scheme. As the role of HPV in VIN lesions was elucidated, subsequent revision by the ISSVD has designated HPV-associated VIN, which comprises the majority of cases, as VIN of the usual type (uVIN) and the less common HPV-independent lesions as VIN, differentiated type (dVIN). The ISSVD has also favored elimination of the VIN 1 category altogether, while other groups have favored a shift from a three tiered system of low-, intermediate- and high-grade lesions to a two-tiered one, eliminating only the intermediate grade.1 This reflects the current understanding that there is no significant biologic or clinical difference between lesions formerly classified as VIN 2 and VIN 3, and that diagnostic reproducibility is much improved in a two tier classification as opposed to a three tiered one. The most recent revision to the terminology has been introduction of the use of a unified terminology for all HPV-related intraepithelial squamous lesions throughout the lower anogenital tract,2 which replaces the previously used "intraepithelial neoplasia (-IN)" term with the term "squamous intraepithelial lesion (SIL)". The World Health Organization (WHO) has supported this terminology but incorporates VIN in parentheses for purposes of clarification.3 In the WHO classification the term vulvar intraepithelial neoplasia, differentiated type (VIN-d) is retained to designate the minority of lesions which are not related to HPV and which do not have a counterpart in other anogenital sites, and these VIN-d lesions are not included in the LSIL/HSIL terminology. The evolution of terminology for vulvar squamous intraepithelial lesions is summarized in Table 1.
Table 1 Summary of evolution of terminology for vulvar squamous intraepithelial lesions
| VIN Terminology (1986) | VIN Terminology (2004) | CAP/ASCCP Terminology (2012) and WHO 2013 |
| VIN 1 | — | Low grade squamous intraepithelial lesion (LSIL/VIN1) |
| VIN 2
|
uVIN | High grade squamous intraepithelial lesion (HSIL/VIN2-3) dVIN |
| VIN 3 | uVIN or dVIN |
Low grade squamous intraepithelial lesions of vulva (LSIL/VIN1)
Although vulvar low grade squamous intraepithelial lesions as well as usual condylomata acuminata of the vulva are caused by infection with human papillomavirus (HPV), usually of low risk (non-oncogenic) types, they are classified separately by WHO by their morphologic appearance. Condylomata acuminata are exophytic verrucoid lesions. When multiple and/or multifocal, a common situation, they are referred to as condylomata acuminata. In contrast low grade squamous intraepithelial lesions (LSIL/VIN1) are typically flat and/or macular-papular. LSIL/VIN1 is a relatively uncommon lesion and not commonly diagnosed. When identified, they are usually found to involve the non-keratinized epithelium of the vestibule, vaginal introitus, or urethra. Both condylomata acuminata and LSIL/VIN1 may involve the vestibule and perianal areas as well as other genital sites. The HPV types most commonly associated with low grade squamous intraepithelial lesions and condyloma acuminatum of the vulva are HPV 6 and 11; however, in a minority of LSIL/VIN1 lesions, and some condyloma acuminatum cases, especially those cases with associated HSIL/VIN2-3, high-risk HPV types may also be found.4, 5 Typical exophytic condyloma acuminatum have a fibrovascular stalk and the epithelial surface is acanthotic and often hyperkeratotic (Figure 1). The fibrovascular stalk is lacking in LSIL/VIN). Both exhibit similar epithelial changes.
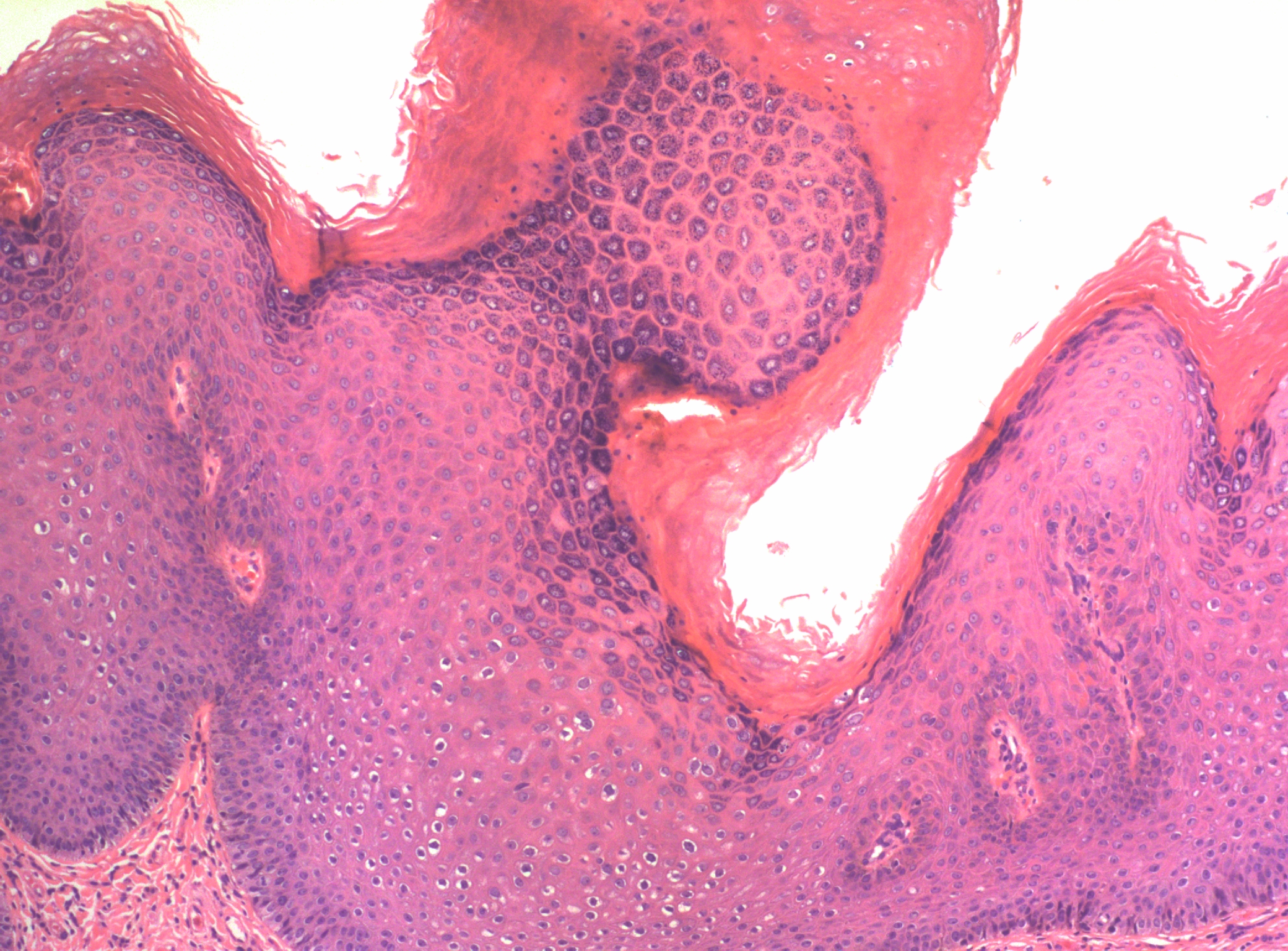 Figure 1 The surface of condyloma acuminatum (LSIL of vulva/VIN 1), showing papillomatosis, acanthosis and hyperkeratosis
Figure 1 The surface of condyloma acuminatum (LSIL of vulva/VIN 1), showing papillomatosis, acanthosis and hyperkeratosis
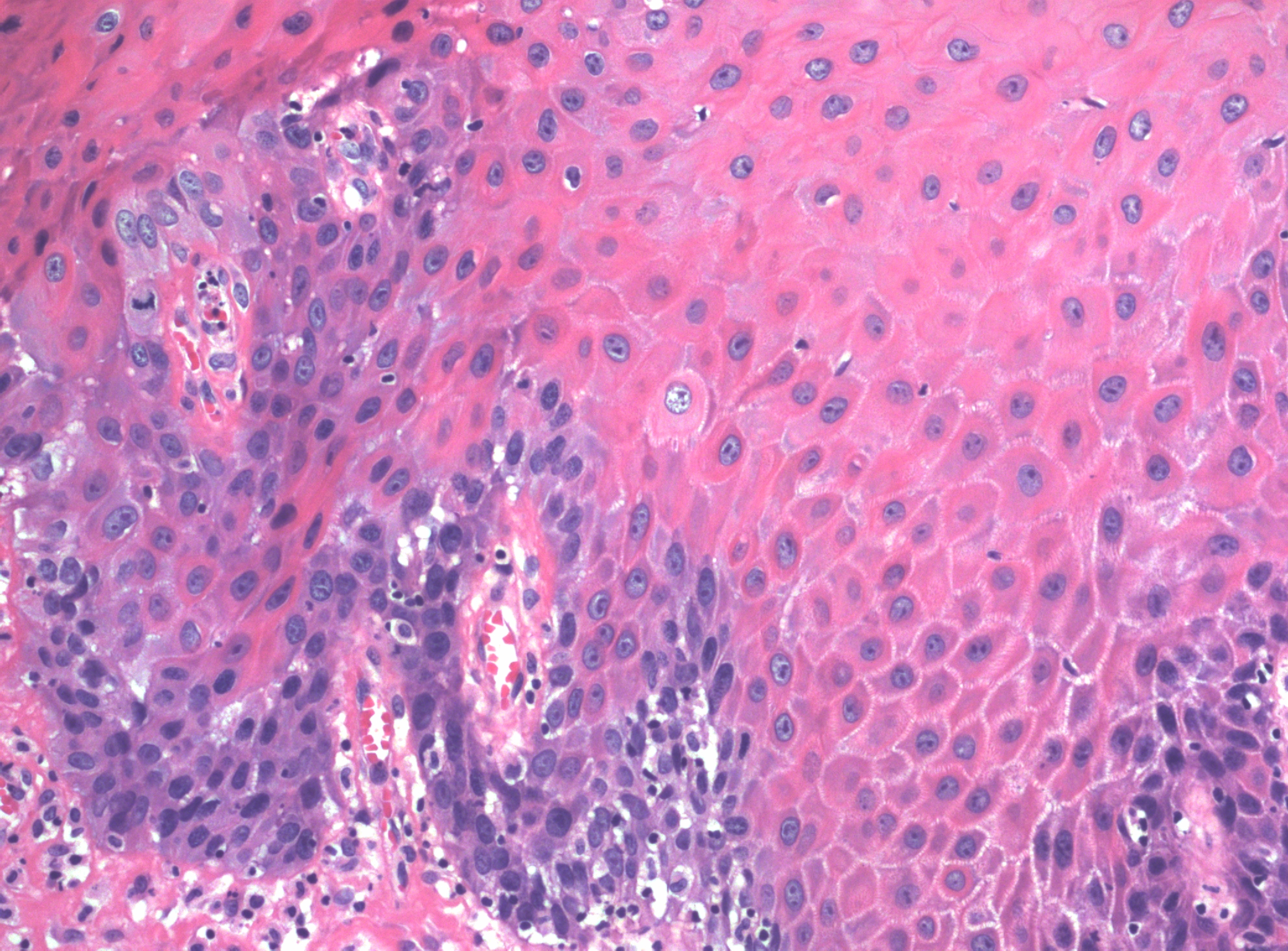
Microscopic features of condyloma acuminatum include a papillomatous lesion with some loss of epithelial maturation from the basal layer to the granular zone. No proliferation above the lower third of the epithelium is seen, but basal and parabasal hyperplasia are usually present with accentuation of the intercellular bridges of the keratinocytes (Figure 2). Individual cell keratinization, dyskeratoses, and increased mitotic figures of normal morphology and location may all be present in varying degrees. Also seen are distinctive koilocytes, which are keratinocytes with distinctive perinuclear clearing and enlarged nuclei that may be hyperchromatic.
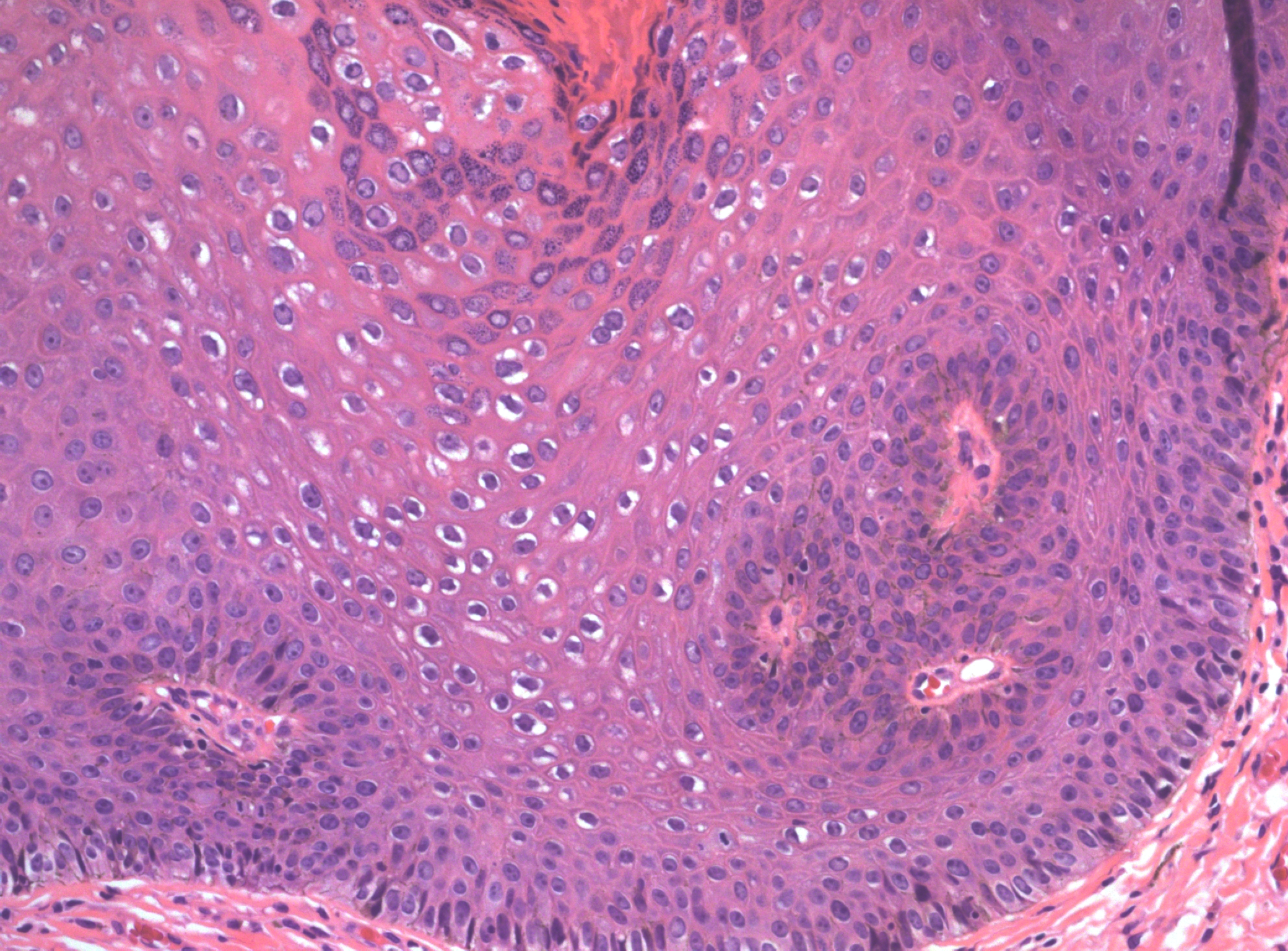 Figure 2 The deeper portion of the condyloma on higher power shows enlargement and irregularity of nuclei with perinuclear clearing (koilocytosis), and prominent intercellular bridges. There is minimal proliferation and immature cells are confined to the basal layers
Figure 2 The deeper portion of the condyloma on higher power shows enlargement and irregularity of nuclei with perinuclear clearing (koilocytosis), and prominent intercellular bridges. There is minimal proliferation and immature cells are confined to the basal layers
The differential diagnoses of condylomata acuminata of the vulva include verrucous carcinoma; vulvar vestibular papillomatosis; condyloma lata of syphilis; and in children other polypoid tumors, including botryoid rhabdomyosarcoma. The diagnosis can be made definitively on histologic examination of biopsy material. The presence of vulvar condyloma acuminatum in children should raise the question of sexual activity or abuse, although HPV infection can be acquired at birth or may be due to cutaneous HPV types.
High grade squamous intraepithelial lesions of the vulva (HSIL/VIN2–3)
It has long been evident that there are two types of intraepithelial lesion of the vulva with potential to develop into malignant disease. The more common type is associated with HPV infection, similar to the high grade squamous intraepithelial lesions seen in the cervix and elsewhere in the lower anogenital tract, and is seen in younger patients. A less common type, known as the vulvar intraepithelial neoplasia, differentiated or (simplex) type, is typically not associated with HPV infection and has a distinct morphology which has no counterpart in any other part of the anogenital tract, and is more commonly found in older patients.
HSIL can occur at any age starting from puberty. The median frequency appears to be among women in the 4th decade of life; this is several decades removed from the peak age of incidence of invasive squamous carcinoma of the vulva. On clinical examination, lesions may be pink, white, red, or brown and typically are slightly elevated (macular) above the level of the surrounding skin. White or acetowhite lesions constitute approximately 70% of all cases, pigmented or red lesions being equally divided at approximately 15% each. Approximately 70% of the cases are multifocal, the remainder being unifocal.6 The most common sites are on the labia minora and the perineum. Perianal involvement occurs in approximately one third of cases of VIN and may extend into the anus. Pruritus is a frequent symptom but is not present in all patients.7, 8, 9 There is a high frequency of cervical and/or vaginal SILs (CIN, VAIN) among patients with SILs of the vulva.7, 8 In particular, HSIL of the vulva is associated with a high frequency of synchronous or metachronous cervical lesions, with oncogenic HPV infection as a common etiologic factor.
Observations that HSIL is frequently found adjacent to tumor in women with invasive squamous cell carcinoma support the concept that HSIL is a precursor of vulvar squamous cell carcinoma. In deeply invasive tumors, HSIL is seen in approximately one-quarter of the cases; however, in women with superficially invasive squamous carcinoma, the frequency of adjacent HSIL approaches 85%. Moreover, 2–28% of patients have been found to have unsuspected invasive carcinoma with a lesion clinically appearing to be HSIL.10 The frequency of progression of HSIL to invasive squamous cell carcinoma is reported under 25%.9, 11, Women with HSIL who are elderly, immunocompromised, or who have Fanconi's anemia are at increased risk for concurrent invasive disease.9 Spontaneous regression of HSIL is well recognized, but long-term studies on these patients are limited, and the rates of recurrence among these women are unknown. When this disease arises during pregnancy, it may spontaneously regress in the postpartum period and require no therapy.12, 13, 14, 15 These uncertainties should be kept in mind when the prognosis and therapy in any individual case of VIN are considered.
Histopathologic features
In normal squamous epithelium, the basal cells divide and thereafter differentiate in a fixed postmitotic fashion. As maturation occurs, cytoplasm is acquired, granules of prekeratin are formed, and the cellular outline and nucleus eventually are lost in the superficial keratin layer. In HSIL, the epithelial cells continue to exhibit large nuclei with nuclear hyperchromasia as they progress upward into the upper two-thirds of the epithelium. These cells are atypical. Mitotic figures are found above the basal and parabasal layers, and are often atypical in appearance. Multinucleation may be seen, which implies impairment of the cell division mechanism. Nuclear pleomorphism, hyperchromasia, and loss of maturation are evidence of abnormal development (Figure 3). The rate of epithelial cell division and the number of cells undergoing cell division may be increased, resulting in an increased density of the cell population.
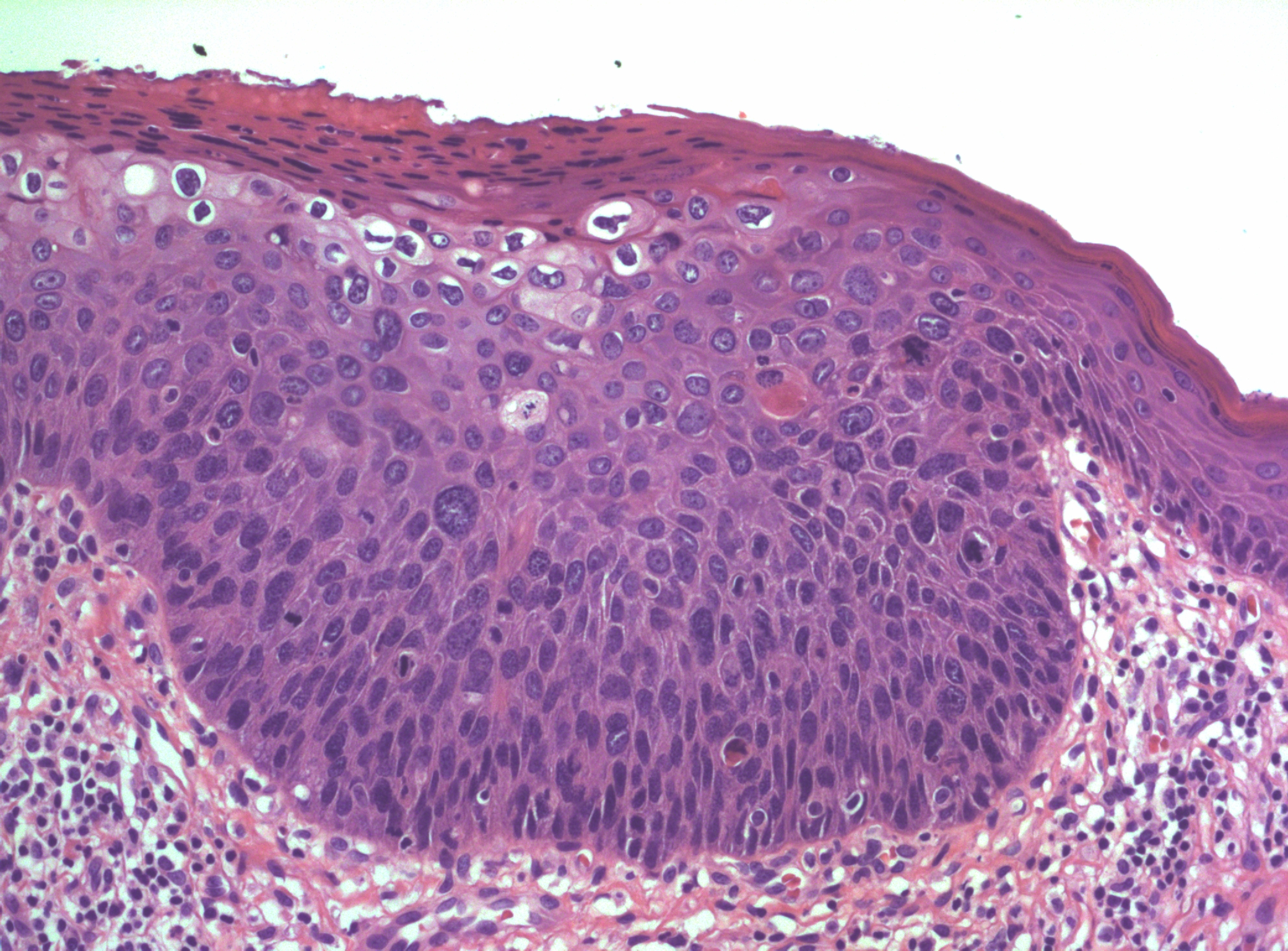 Figure 3 High grade squamous intraepithelial lesion of vulva (HSIL/VIN3) with adjacent normal epithelium on the right edge. The lesion is acanthotic with pronounced nuclear crowding and atypia. Koilocytic change is seen towards the surface. Numerous mitotic figures can be seen in both the basal third and upper layers of the epithelium. There is lack of maturation extending well above the basal third as well.
Figure 3 High grade squamous intraepithelial lesion of vulva (HSIL/VIN3) with adjacent normal epithelium on the right edge. The lesion is acanthotic with pronounced nuclear crowding and atypia. Koilocytic change is seen towards the surface. Numerous mitotic figures can be seen in both the basal third and upper layers of the epithelium. There is lack of maturation extending well above the basal third as well.
The presence of a granular layer, or keratinized surface, is not an indication to downgrade an HSIL. On the vulva, the superficial layers of cells are either keratinized or parakeratotic, so a “full-thickness” change, per se, rarely exists.
There may be a variety of microscopic features in HSIL of the vulva. HPV-related lesions may be of the basaloid type or the warty type, or some combination of the two. Basaloid lesions are characterized by relatively uniform cells without significant maturation from the basal-parabasal cells (Figure 4), while warty lesions resemble condyloma acuminatum, with koilocytosis, hyperkeratosis, and dyskeratosis (Figures 5 and 6). Differentiated (simplex) VIN (VIN–d) lesions have prominent eosinophilic cytoplasm in the parabasal layer and exhibit keratin formation (Figures 7 and 8).12, 16, 17, 18
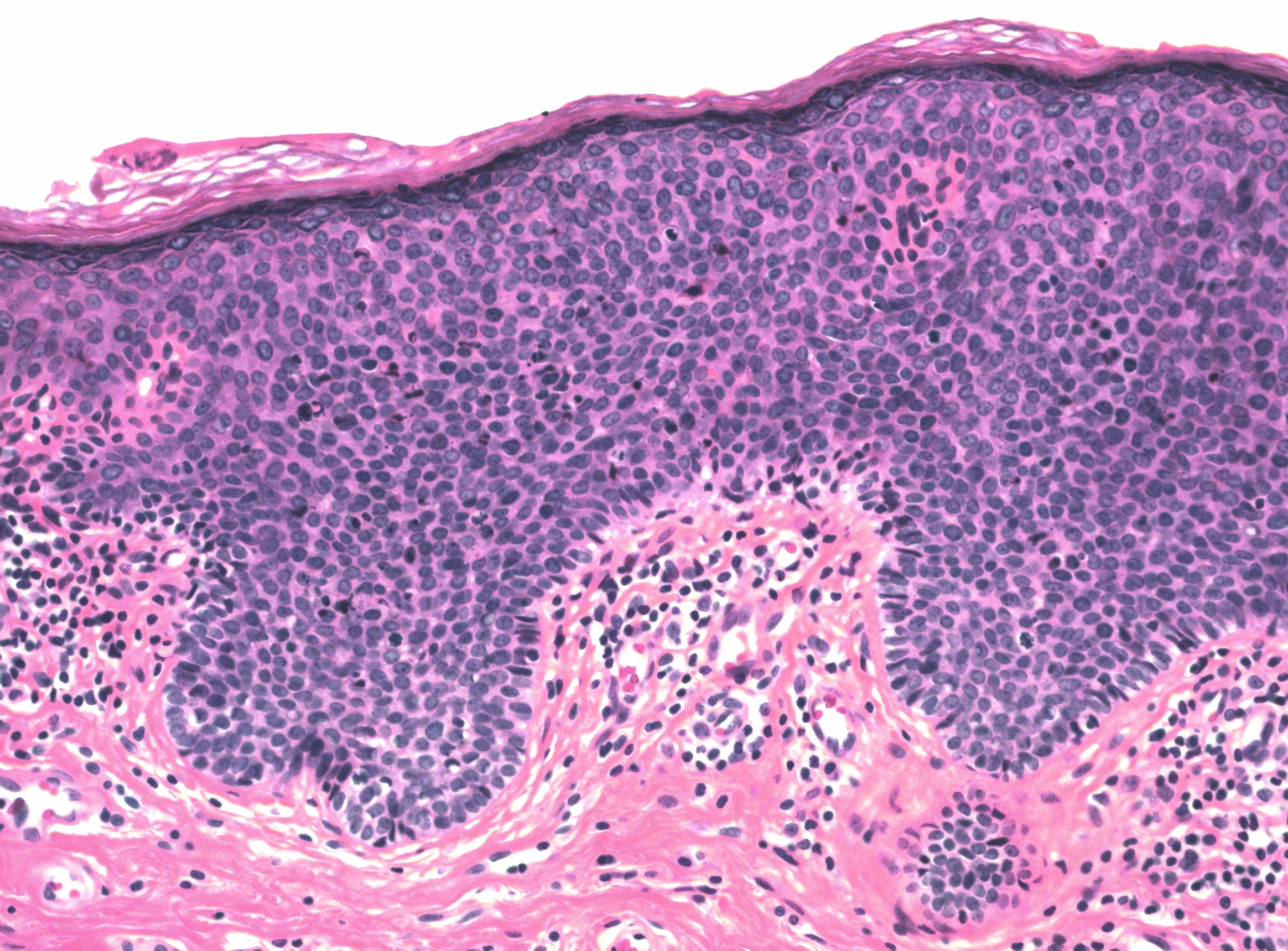 Figure 4 Basaloid HSIL of vulva (VIN 3). The full thickness of the epithelium is replaced with immature-appearing cells with high nuclear to cytoplasmic ratios
Figure 4 Basaloid HSIL of vulva (VIN 3). The full thickness of the epithelium is replaced with immature-appearing cells with high nuclear to cytoplasmic ratios
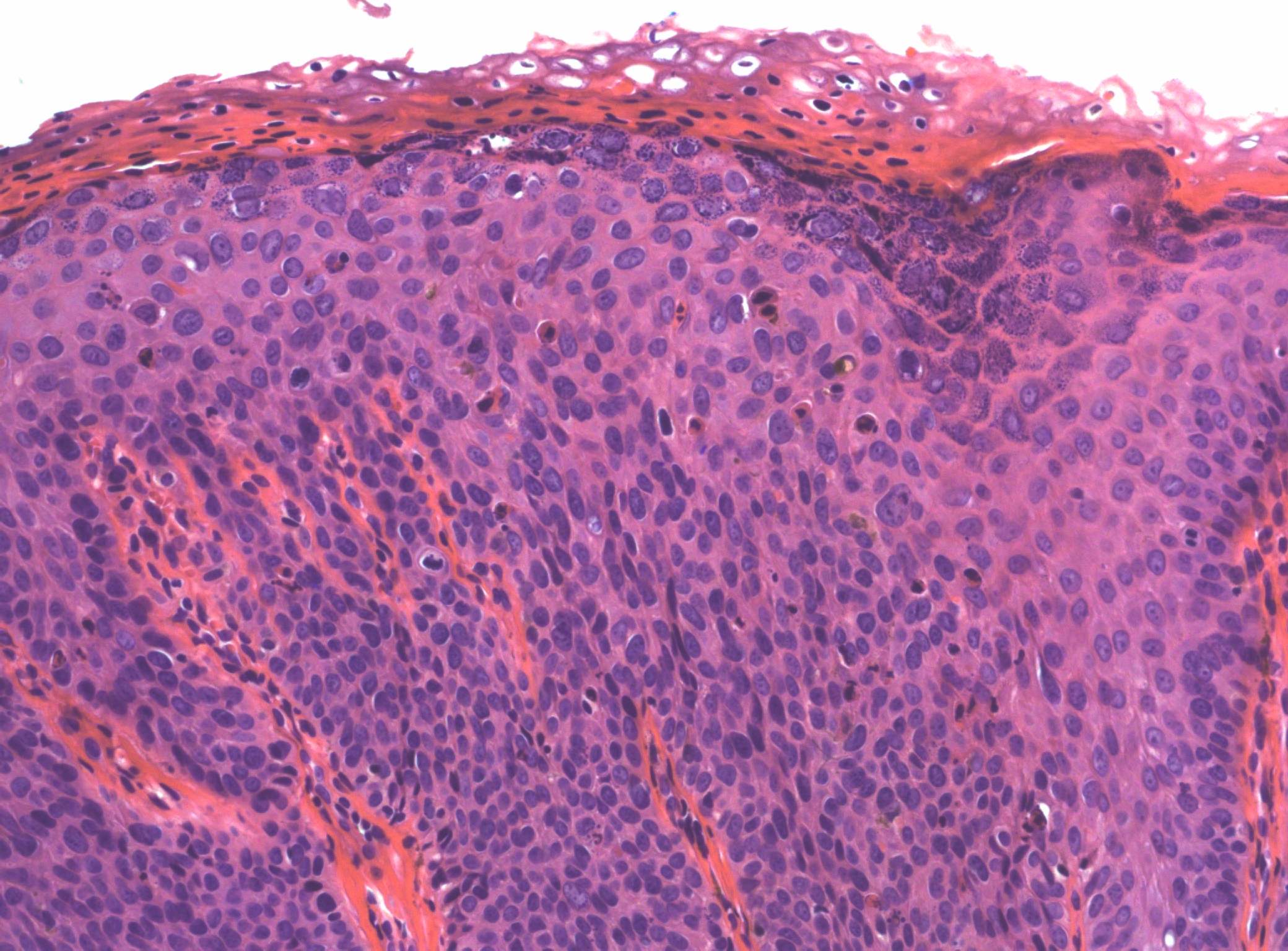 Figure 5 Warty HSIL of vulva (VIN 3). Immature cells are prominent through almost the full thickness of the epithelium, but there is some maturation towards the surface with hypergranulosis, parakeratosis and koilocytic change. Numerous dyskeratotic cells are present throughout the lesion
Figure 5 Warty HSIL of vulva (VIN 3). Immature cells are prominent through almost the full thickness of the epithelium, but there is some maturation towards the surface with hypergranulosis, parakeratosis and koilocytic change. Numerous dyskeratotic cells are present throughout the lesion
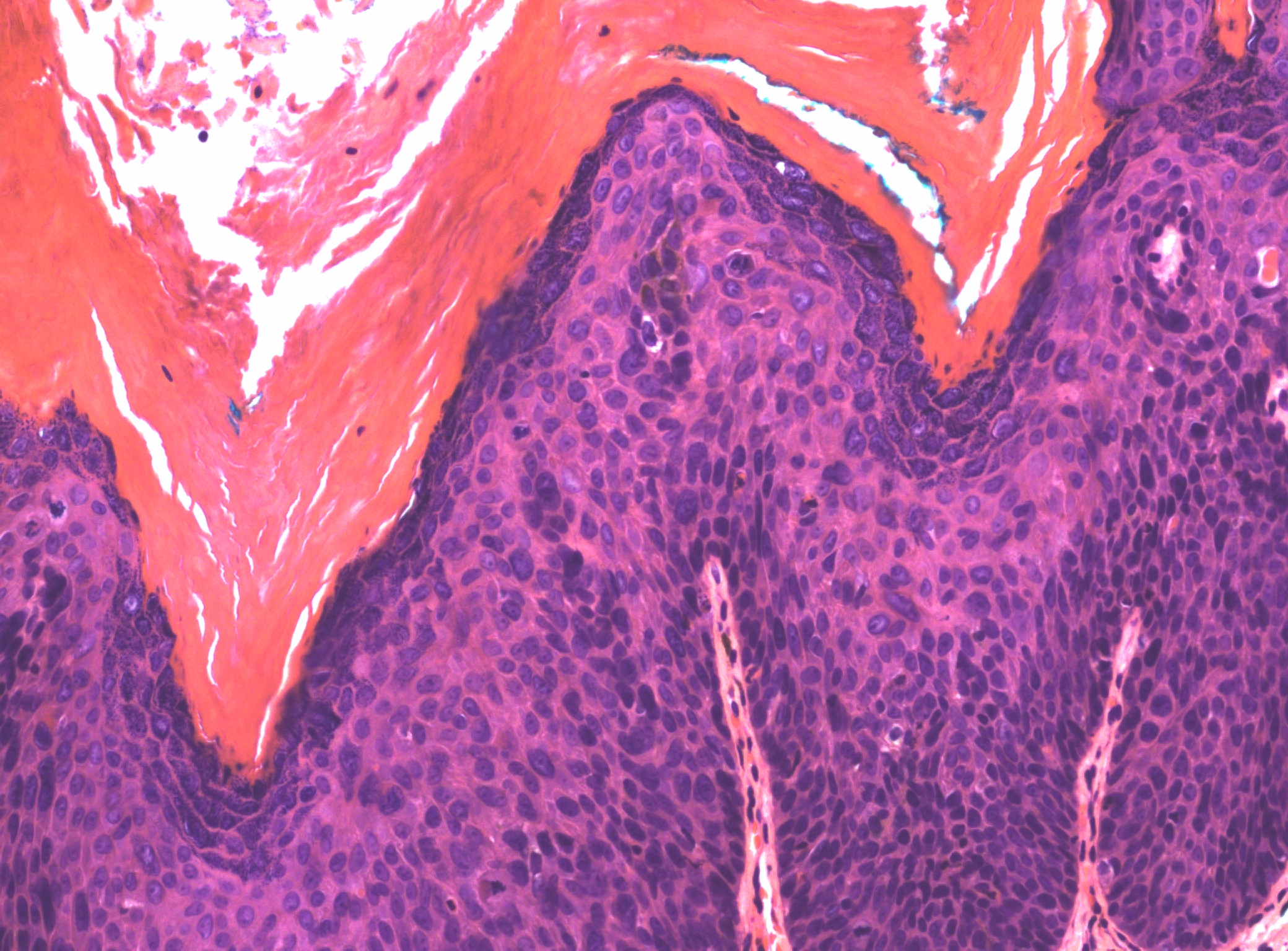 Figure 6 The surface of this warty HSIL shows a spiky architecture, and there is marked hyperkeratosis
Figure 6 The surface of this warty HSIL shows a spiky architecture, and there is marked hyperkeratosis
Figure 7 VIN, differentiated type (d-VIN). There is atypia of the basal layers and premature keratinization of the suprabasal layers, with abundant eosinophilic cytoplasm and prominent cell borders
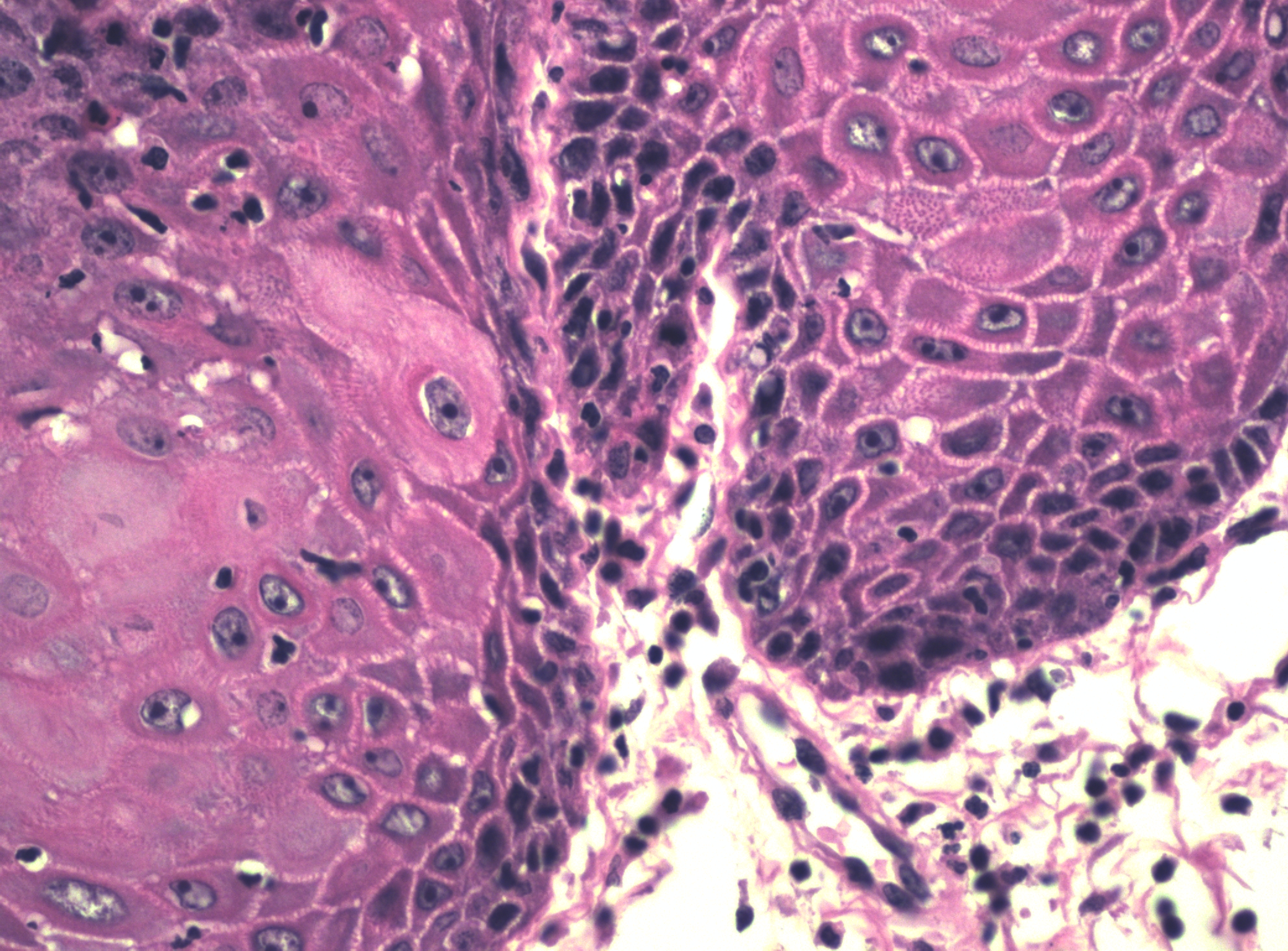 Figure 8 A higher power of VIN, differentiated type (d-VIN)
Figure 8 A higher power of VIN, differentiated type (d-VIN)
HPV has been identified by Southern blot, in situ hybridization, and polymerase chain reaction in more than 80% of HSIL lesions studied, corresponding to the basaloid and warty types.19 The variety of HPV types found in preinvasive cervical lesions has not been observed in the vulvar intraepithelial lesions. HPV 16 is the most common type found in the vulva. Because they are HPV related, most HSIL basaloid and warty lesions express p16INK4a. Differentiated VIN is typically not HPV related but is associated with lichen sclerosus.12, 20 Differentiated VIN (VIN-d) lesions typically do not express p16INK4a by immunohistochemistry, but may express p53 (see section on squamous cell carcinoma, microscopic features, below).16, 17
In pigmented HSIL lesions, there is a concentration of melanin in the dermal melanophages and basal keratinocytes. The presence or absence of pigmentation does not appear to be associated with clinical behavior but rather location on the vulva, with those VIN lesions involving the skin being more commonly pigmented than those involving the vulvar mucosa of the vestibule. Most lesions show an inflammatory response beneath the disordered epithelium.
Treatment
HPV-related squamous intraepithelial lesions in other areas may precede, coincide with, or follow the onset of the vulvar HSIL lesion. Patients with basaloid or warty types of HSIL are considered to be in a high-risk category for cervical and vaginal SIL and must receive follow-up, including cervical Pap testing and oncogenic HPV testing, with follow-up appropriate colposcopy when the cytologic findings are abnormal. HSIL may involve the perianal skin and extend into the anal–rectal mucosa. This is well documented and is not specifically related to anoreceptive sexual exposure. Should HSIL be seen in the perianal area, anoscopy, with anal cytology and appropriate biopsies, if a lesion is identified, should be considered to determine the extent of the disease and plan appropriate management.
Therapy for HSIL should be administered on an individualized basis. Total superficial or deep vulvectomy, as has been advocated in the past, constitutes overtreatment. Many patients with this disease are young and sexually active. Every attempt should be made to retain a functioning clitoris and vulva and to provide an acceptable functional and cosmetic result. HSIL is, by definition, confined to the epithelium. This permits sparing of the deeper tissues.
Lesions confined to the inner vulvar vestibule surfaces can be treated with superficial excision or laser ablation, provided that prior biopsy has excluded invasion. Superficial local excision with a 1-cm margin can be performed on localized lesions. Larger lesions can be treated with local excision with or without laser ablation. Topical treatment with imiquimod cream has also been reported as effective provided invasive carcinoma has been excluded. Histologic evaluation of the specimen, when excision is performed, is necessary to evaluate for invasion and to define extent of the lesion and the status of the margins of resection. Frozen sections at the time of surgery are not necessary and only add to the cost of treatment. With uninvolved margins of resection, and absence of occult invasive foci within the lesion itself, the prognosis for patients treated in this manner is extremely good. Should new foci of disease occur, they can be handled in a similar fashion. The rate of local recurrence is approximately 6% for patients with prior negative margins and approximately 20% for patients with involved margins.10 Should a margin be indeterminate, such as occurs when surface epithelium is lost or involved with LSIL, re-excision of the involved margins is not usually necessary. Even if a margin is involved with HSIL, re-excision may not be needed and a follow-up examination can determine whether HSIL is present and further treatment necessary.
Extensive confluent multicentric lesions involving the entire vulva demand a careful treatment plan. Multicentric lesions can be treated with superficial excision alone or with a combination of superficial excision and laser ablation.21 Topical therapy with imiquimod may also be considered for smaller and multiple lesions. It is recognized that skin appendage involvement by HSIL occurs and, in hair-bearing skin, may involve skin appendages to a depth of up to 2.9 mm. In non-hair bearing skin (e.g., vulvar vestibule, labia minora, clitoris, perineal body), skin appendage involvement is not an issue, and in these areas, the lesion is typically less than 1-mm thick.22 Consequently, a plan of therapy should use a local superficial excision of the lesions involving the hair-bearing skin and the skin with sebaceous glands without hair (e.g., medial labia majora, perianal and perineal areas peripheral to Hart's line).20 Superficial excision “skinning” procedures (partial superficial vulvectomy) are one approach for such cases. As a rule, the surgical wound edges can be approximated primarily by mobilization of vaginal and vulvar epithelium. Creation of full-thickness flaps of adjacent skin may be applicable in selected cases where wound closure needed. In such cases some surgeons prefer the primary grafting of the denuded vulvar area with skin removed from a thigh donor site. Treatment with laser ablation, or superficial excision, of HSIL involving the clitoris, vestibule, labia minora, and perineal body proximal to Hart's line (i.e., the junction between the nonkeratinized epithelium of the vestibule and the skin) is an effective approach. In these non-hair bearing areas, it is essential that careful evaluation and selective biopsy of the lesions be performed to exclude associated invasive carcinoma before treatment is initiated.23
Either technique gives a good cosmetic result and extremely low recurrence rate, provided the initial procedure is carried down to a level well below the epidermis and its adnexa and beyond the visible margins of disease. The clitoris usually can be spared, thus retaining the possibility of function. Irradiation and radical surgical excision have no place in the treatment of HSIL.
A variety of topical methods have demonstrated some usefulness in HSIL. Topical 5-fluorouracil has been advocated in the past. Therapy consists of topical application of a 5% 5-fluorouracil cream applied three times per week for a period of 6 weeks. This therapy is not currently recommended in women of reproductive age because of changes in the US Food and Drug Administration's position regarding this medication and its potential side effects in pregnancy.
Some studies have shown promising results using photodynamic therapy (PDT), particularly 5-aminolevulinic acid (5-ALA), as a nonsurgical approach to the treatment of HSIL. In one study of 25 patients24 using topical sensitization of lesions with 5-ALA and subsequent PDT, all low grade lesions showed responsiveness, as did all unifocal and bifocal lesions regardless of grade. However, multifocal HSIL did not respond well to the treatment. Use of topical imiquimod, an immune response modifier, for HSIL has shown promising results.25, 26
PAGET DISEASE
Paget disease of the vulva is an intraepithelial neoplastic process; however, it is a unique and biologically distinct entity with specific morphologic characteristics and oncologic implications that warrant separate discussion. Paget disease of the skin was first described by Sir James Paget in 1874 in the nipple and areola of the breast and was associated with an underlying breast carcinoma (mammary Paget disease).
Extramammary Paget disease can arise in the female lower genital tract within the vulva or perianal area. Most cases of extramammary Paget disease of the vulva or perianal region are primary to these sites; that is, they arise from within the vulvar or perianal epithelium. However, not all cases of vulvar or perianal Paget disease are primary in origin, and a small percentage of cases are actually manifestations of an internal malignancy (most commonly anorectal adenocarcinoma or urothelial carcinoma from the bladder or urethra) that has spread or metastasized within the epithelium to secondarily involve the vulva or anus. Secondary Paget disease of the vulva constitutes approximately 12% of vulvar Paget cases.
It is essential to distinguish primary from secondary vulvar Paget disease, because of the difference in treatment for the two entities, the classification scheme of Wilkinson and Brown based on cell of origin has been proposed (Table 2). It is generally accepted that the Paget cells of primary cutaneous type (type 1) arise from an intraepidermal, pluripotent stem cell with apocrine gland differentiation.
Table 2. Subtypes of vulvar Paget disease
| Type 1 | Paget disease as a primary cutaneous neoplasm |
| Type 1a | Paget disease as a primary intraepithelial neoplasm |
| Type 1b | Paget disease as an intraepithelial neoplasm with invasion |
| Type 1c | Paget disease as a manifestation of an underlying primary adenocarcinoma of skin, appendage, or subcutaneous gland |
| Type 2 | Paget disease secondary to adenocarcinoma of nonskin origin, including anal or rectal adenocarcinoma |
| Type 3 | Paget disease secondary to urothelial neoplasia; pagetoid urothelial intraepithelial neoplasia (PUIN) |
Virtually confined to postmenopausal white women, primary cutaneous vulvar Paget disease appears as a reddish-pink area interlaced with patches of hyperkeratotic white epithelium, usually on the lateral aspects of the labia majora. The appearance is not unlike that of a severe cutaneous candidiasis or eczema, and the disease is often confused with these benign conditions. The reluctance to perform a simple office biopsy often leads to delay in diagnosis. The disease begins as a localized process, but it can involve the entire vulva, perianal skin, and inner thigh regions. It is not uncommon for the visible margins of the disease to not correspond to the extent of histologic involvement.
In contrast, vulvar Paget disease as a manifestation of anal or rectal adenocarcinoma (type 2) typically involves the perianal skin, as well as adjacent, contiguous vulvar skin, and as a manifestation of urothelial carcinoma (type 3) primarily involves the vulvar vestibule and paraurethra. However, these cases of secondary Paget disease appear as eczematoid lesions, similar to primary vulvar Paget disease, and therefore, histologic examination is necessary to separate them. A summary of the clinical manifestations of Paget disease types 1, 2, and 3 is provided in Table 3.
Table 3. Clinical manifestations of subtypes of vulvar Paget disease
| Type | Clinical presentation |
| Primary cutaneous vulvar Paget disease (type 1) | Presents on vulvar skin, often on the lateral aspects of the labia majora |
| Secondary Paget disease as a manifestation of anal or rectal adenocarcinoma (type 2) | Typically involves the perianal skin, as well as adjacent, contiguous vulvar skin |
| Secondary Paget disease as a manifestation of urothelial carcinoma (PUIN) (type 3) | Primarily involves the vulvar vestibule and periurethral vulvar epithelium |
From Wilkinson EJ, Brown HM: Pagetoid urothelial intra-epithelial neoplasia (PUIN): Differentiation from Paget disease of cutaneous or ano-rectal origin. Mod Pathol 14(1)147A, 2001; Wilkinson EJ, Brown HM: Vulvar Paget Disease of Urothelial Origin: A report of three cases and a proposed classification of vulvar Paget disease. Hum Pathol 2002;33(5):549-554.
Histopathologic features
Microscopically, in primary cutaneous (type 1) Paget disease, the involved skin contains groups of large, pale cells that are round to oval (Figure 9). In secondary Paget disease, secondary to spread from urothelial carcinoma, the cells tend to be smaller and more hyperchromatic than in type 1, but distinguishing the different types microscopically without the use of additional stains can be very difficult. The Paget cells, in primary cutaneous (type 1) or secondary anorectal (type 2) Paget disease, have abundant cytoplasm rich in neutral and acidic mucopolysaccharides. Therefore, such cells have characteristic staining reactions, and their cytoplasm exhibits positive staining with periodic acid-Schiff (PAS, diastase-resistant) and mucicarmine. Additional immunohistochemical studies are useful in distinguishing primary from secondary Paget disease, as well as distinguishing Paget and PUIN from other intraepithelial neoplastic processes (Table 4).
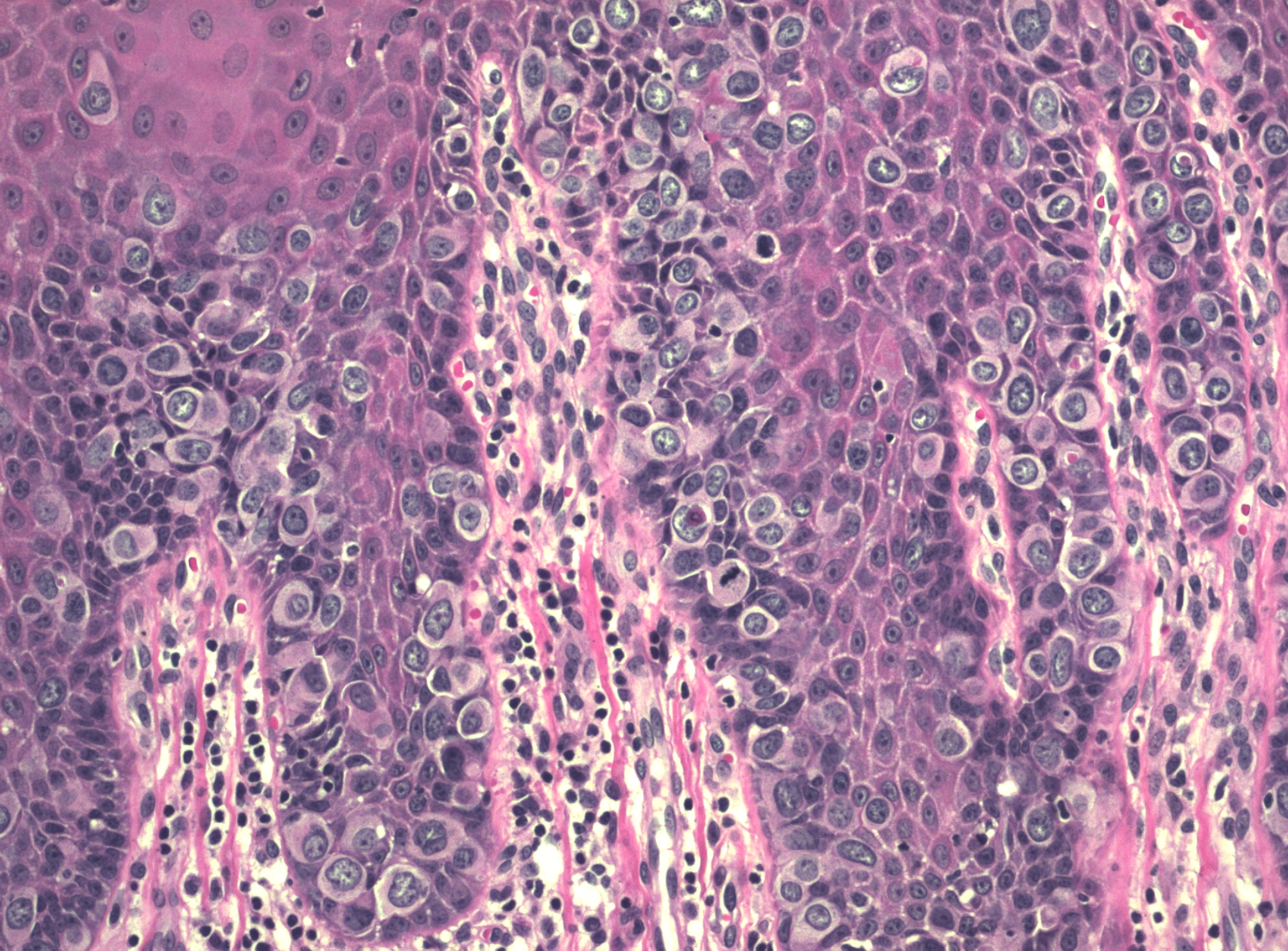 Figure 9 Paget disease of vulva. The abundant round cells with pale cytoplasm are most evident in the basal layers of the epithelium in this case
Figure 9 Paget disease of vulva. The abundant round cells with pale cytoplasm are most evident in the basal layers of the epithelium in this case
Table 4. Summary of the immunohistochemical findings of primary and secondary vulvar Paget disease
|
| CK 7 | CK 20 | GCDFP | CEA | UP-III |
| Primary | + | +/− | +/− | + | − |
| Secondary to anorectal adenocarcinoma | − (rare +) | + | − | + | − |
| Secondary to urothelial carcinoma | + | +/− | − | +/− | + |
CK 7, cytokeratin 7; CK 20, cytokeratin 20; GCDFP, gross cystic disease fluid protein; CEA, carcinoembryonic antigen; UP-III, uroplakin-3. Adapted from Wilkinson EJ, Brown HM: Vulvar Paget disease of urothelial origin: a report of three cases and a proposed classification of vulvar Paget disease. Hum Pathol 33: 549, 2002.
The characteristic Paget cells usually are found singly or in nests along the basal layer of the epithelium, and they may extend to involve the sweat gland and hair shaft epithelia. This involvement of skin appendages should be distinguished from invasion. In most cases, Paget disease is entirely intraepithelial; however, there is a subgroup of cases in which associated invasive adenocarcinoma is found. Approximately 20% of patients with vulvar Paget disease have sweat gland or Bartholin's gland adenocarcinoma beneath the clinically recognizable Paget disease.27
Clinical behavior and treatment of vulvar Paget disease
Primary intraepithelial cutaneous vulvar Paget disease is a chronic, indolent process confined by the basement membrane and marked by recurrences. Invasive primary Paget disease has been observed contiguous to typical intraepithelial Paget disease in 10–20%. The prognosis in cases with underlying invasive disease is poor. Therefore, the primary approach to primary Paget disease should include surgical excision to the deep fascia to exclude underlying invasion.28 Excision should include all epithelium visibly involved with Paget disease, with a margin as wide as possible (a 1–2-cm border of apparently normal skin is not excessive). If the initial surgery shows underlying adenocarcinoma, partial or total vulvectomy with ipsilateral inguinal-femoral lymphadenectomy is indicated as a secondary procedure.
Despite generous surgical margins, in many cases the margins may prove to be microscopically positive, and recurrences along the lines of wound approximation are common, even with negative surgical margins. When the entire visible Paget lesion is excised, with a 1-cm margin, performing frozen sections for assessment of margins may not be as useful as sometimes presumed in that the presence or absence of intraepithelial Paget disease at the margins does not greatly influence the risk of recurrence recognizing that approximately one third of cases with negative margins will have recurrent intraepithelial Paget disease.29 Recurrences have been reported in skin grafts applied to the surgical defect, along and across suture lines, and in contiguous areas of “normal” skin. Extensive radical vulvar surgery to excise intraepithelial Paget disease is not desirable when vulvar sparing treatment is available. Because the danger of subjacent cancer already is ruled out by the primary surgery, the risk of invasive tumor in nonprimary clinically normal-appearing areas with intraepithelial Paget disease is remote, and radical excision of these areas has not been proved to reduce recurrence.30 The treatment of such recurrent lesions can be focused on the intraepithelial disease, and topical therapy is useful. The application of topical 5% 5-fluorouracil cream three times weekly for 6 consecutive weeks, as well as topical imiquimod, or topical bleomycin have been used to successfully treat recurrence; however, laser ablation and local excision are both effective treatments in peripheral sites.20, 23, 24, 25, 31, 32
Surgical treatment of secondary vulvar Paget disease should be different to that of primary vulvar disease. Vulvar Paget disease related to anal or rectal adenocarcinoma or urothelial carcinoma is usually entirely intraepithelial within the vulva. Therefore, the treatment for secondary vulvar Paget disease can be modified to address the primary neoplastic anal or rectal lesion and treat the intraepithelial vulvar disease as an intraepithelial, not an invasive, process.27 In most cases, the superficial involvement of the vulvar skin can be treated with superficial excision without having to extend to the fascia or with other superficial ablative techniques.27
In summary, vulvar Paget disease, from a surgical standpoint, should be viewed as either primary or secondary to the vulva because of the differences in treatment and tumor biology (Table 5).
Table 5. Etiologic classification of extramammary genital Paget disease
| Primary Paget disease (cutaneous origin) (type 1)* |
| Intraepithelial Paget disease (type 1a) |
| Intraepithelial Paget disease with invasion (type 1b) |
| Intraepithelial Paget disease with associated skin appendage adenocarcinoma (type 1c) |
| Secondary Paget disease (noncutaneous origin) |
| A manifestation of associated adenocarcinoma (type 2), including: |
| Anal/rectal/colonic |
| Cervical |
| Other |
| A manifestation of urothelial carcinoma/PUIN (type 3) |
PUIN, Pagetoid urothelial intraepithelial neoplasia.
*Other sites reported include axillary, eyelid, external ear canal, umbilical, inguinal femoral skin, etc. Adapted from Wilkinson EJ, Brown HM: Vulvar Paget disease of urothelial origin: a report of three cases and a proposed classification of vulvar Paget disease. Hum Pathol 33: 549, 2002; Pierie JP, Choudry U, Muzikansky A, Finkelstein DM, Ott MJ. Prognosis and management of extramammary Paget's disease and the association with secondary malignancies. J Am Coll Surg 196(1): 45−50, 2003.
SQUAMOUS CELL CARCINOMA
Squamous cell carcinoma is the most common malignant tumor of the vulva, constituting more than 90% of all vulvar malignancies and accounting for approximately 4% of all genital malignancies in women. There is evidence that the incidence of vulvar carcinoma is rising.33 The frequency of this disease increases with age, and it is most common in women older than 75 years in whom its incidence is 19.9 per 100,000 women.22 Vulvar squamous cell carcinoma is, however, well documented in young women.17, 34
Epidemiologic studies support the hypothesis that vulvar carcinoma has at least two origins: one oncogenic HPV-related and one not related to HPV. In younger patients, these lesions are frequently HPV-related with VIN as an apparent precursor. The VIN lesions are usually of the warty or basaloid type and are usually associated with basaloid or warty types of squamous cell carcinoma. These women are often heavy cigarette smokers. Cigarette smoking alone appears to increase the odds ratio. The other group of women is older and does not appear to have HPV-related vulvar lesions, nor does there appear to be an association with cigarette smoking in this group. This older group of women may have associated vulvar dermatoses, usually lichen sclerosus. Other risk factors for vulvar carcinoma in all groups include carcinogen exposure, immunosuppression, chronic granulomatous disease, and prior cervical or vaginal carcinoma. The association between squamous cell carcinoma of the cervix or vagina and squamous carcinoma of the vulva is well established. As in VIN lesions, oncogenic HPV infection is a common etiologic factor leading to multifocal genital neoplasia.35 Obesity and poor perineal hygiene also are implicated in many cases. Pregnancy and high parity do not appear to be associated.
Clinical manifestations
Squamous carcinoma of the vulva usually appears as an ulcerated, exophytic, or indurated mass that may be associated with pruritus, local infection, pain, foul odor, and/or bleeding.20 The tumor may be identified anywhere on the vulva but the labia majora, labia minora, and clitoris are the most common sites. The clitoris is identified as the primary site in 5–15% of cases.
Squamous cell carcinoma of the vulva is typically divided into two categories: (1) superficially invasive squamous cell carcinoma and (2) frankly invasive squamous cell carcinoma. VIN may be found adjacent to invasive carcinoma in approximately 60% of cases and is found more commonly among younger women and in those with more superficially invasive tumors. Conversely, superficially invasive vulvar carcinoma may be encountered in the treatment of VIN. Vulvar lichen sclerosus coexists with carcinoma in approximately 15–40% of cases.22 Although squamous cell hyperplasia has been found at the edges of invading tumor, it is most probable that the hyperplastic lesions seen immediately adjacent to squamous cell carcinomas are induced by factors associated with the carcinoma, or by the carcinoma itself, and may not necessarily be a precursor lesion. The tissue adjacent to the tumor may harbor VIN, lichen sclerosus, or nonspecific hyperplasia or may appear normal.36 In most cases of vulvar carcinoma, the tumor occurs within a single focus, usually within the clitoris, perineal body, or medial aspects of the labia majora or minora.
Squamous carcinoma of the vulva is generally slow growing and spreads by extension to contiguous skin, vagina, and rectum. The lesion may also invade deeper tissues and become fixed to the pelvic bones. Metastases to lymph nodes involve the femoral and inguinal nodes before they progress to the deep obturator, internal lilac, or periaortic group. When nodal metastases occur, the tumor typically involves the ipsilateral nodes and, in rare cases, the contralateral nodes. When the tumor is midline, bilateral nodal involvement may occur.37 The current clinical staging system for vulvar carcinoma was defined in 1995 by FIGO. The International TNM staging is also commonly used (Table 6).38 The system no longer relies on the clinical assessment of groin nodes, a procedure that can be quite inaccurate, based on a comparison of clinical findings with surgical and pathologic findings.39
Table 6. Vulvar carcinoma staging: comparison of the AJCC and FIGO nomenclatures, TNM stage groupings, and correlation with FIGO staging
|
AJCC | T | N | M | FIGO |
| Stage 0 | Tis | N0 | M0 |
|
| Stage I | T1 | N0 | M0 | Stage I |
| Stage IA | T1a | N0 | M0 |
|
| Stage IB | T1b | N0 | M0 |
|
| Stage II | T2 | N0 | M0 | Stage II |
| Stage III | T1 | N1 | M0 | Stage III |
|
| T2 | N1 | M0 |
|
|
| T3 | N0,N1 | M0 |
|
| Stage IVA | T1 | N2 | M0 | Stage IVA |
|
| T2 | N2 | M0 |
|
|
| T3 | N2 | M0 |
|
|
| T4 | Any N | M0 |
|
| Stage IVB | Any T | Any N | M1 | Stage IVB |
Adapted from AJCC, American Joint Commission on Cancer.
FIGO, International Federation of Gynecology and Obstetrics.
Histopathologic types of vulvar squamous cell carcinoma
Vulvar squamous cell carcinomas can be considered as one of two groups, specifically as associated with oncogenic HPV or not associated. HPV type 16 is the most common type identified. Those tumors associated with HPV are infrequent in older women, identified in approximately one-fifth of vulvar carcinomas in older women (mean age 77 years); whereas over two-thirds of squamous carcinomas of the vulva in younger women are HPV associated. (age 50 years). The histopathologic features of the invasive carcinomas also differ in these two groups, with tumors of older women having squamous cell carcinomas that are well differentiated and keratinized. In younger women the squamous carcinomas are usually basaloid or warty/ condylomatous types, tumor types associated with HPV.40, 41
In addition to the more common squamous cell carcinoma histopathologic types, including keratinizing squamous cell carcinoma, basaloid carcinoma, and warty carcinoma, a number of far less common squamous cell carcinomas are recognized that have distinctive histology. These are important because they need to be differentiated as some like giant cell carcinoma have a worse prognosis, and some may resemble melanoma, adenocarcinoma, or lymphoproliferative or soft tissue tumors. These tumor types are listed below, but the reader is referred to gynecologic pathology texts for discussion.4, 26, 42
GIANT CELL SQUAMOUS CARCINOMA
Squamous cell carcinoma with tumor giant cells is characterized by multinucleated epithelial tumor giant cells with large nuclei, prominent nucleoli, and little cytoplasm. This tumor variant is relatively rare and is associated with a poor prognosis.43
SPINDLE CELL SQUAMOUS CELL CARCINOMA
Spindle cell carcinoma of the vulva may induce a spindle cell dermal reaction and mimic a sarcoma or spindle cell melanoma.44
ACANTHOLYTIC SQUAMOUS CELL CARCINOMA
This tumor in the vulva has also been referred to as adenoid squamous carcinoma, adenocanthoma, and adenosquamous carcinoma.45 It is considered to arise in the vulva as a primary skin appendage tumor and should be distinguished from a metastatic carcinoma. In some series this tumor has a poorer prognosis than the usual squamous cell carcinomas, although experience is limited.
PAPILLARY SQUAMOUS CELL CARCINOMA
This tumor is rare and has similar morphologic features to papillary squamous cell carcinoma of the cervix. The tumor is exophytic with an expansile and deep pushing infiltrative pattern within the dermis. Although there is very limited experience with this tumor, with negative nodes it can be managed by deep wide excision.46
LYMPHOEPITHELIOMA-LIKE CARCINOMA
These tumors may occur rarely on the vulva in older individuals.47 They are composed of nests or syncytial groups of epithelioid-appearing cells mixed with, and surrounded by, a dense lymphocytic infiltrate.
PLASMACYTOID SQUAMOUS CARCINOMA
A rare tumor of the vulva presents as a submucosal mass composed of plasmacytoid appearing neoplastic squamous cells that must be distinguished from malignant plasma cells (plasmacytoma) or other similar appearing tumors. Although there is very limited experience the tumor has been reported to be very aggressive.48
The prognosis for vulvar carcinoma is primarily related to the size of the original lesion and the presence or absence of nodal metastases. Patients with tumors confined to the vulva with tumor-free groin nodes have a corrected 5-year survival rate of 90% after adequate excision of the tumor and usual ipsilateral inguinal-femoral lymphadenectomy. If the tumor has spread to the groin nodes, the corrected 5-year survival rate decreases to 65%. If the tumor has spread to involve the pelvic nodes as well, the survival rate is less than 25%.22, 36, 49 Large squamous carcinomas of the vulva have been reported in association with hypercalcemia, without bone metastasis.50 This is primarily mediated through the production of parathyroid hormone from the neoplastic keratinocytes.49, In addition, attempts at medical correction of the hypercalcemia have been unsuccessful. After excision of the tumor, the serum calcium level returns to normal but rises again with recurrent disease.
STAGE IA INVASIVE CARCINOMA OF THE VULVA
The concept of superficially invasive carcinoma of the vulva implies that lesions with limited dermal invasion may constitute a separate prognostic category.48, 49, 51 The term microinvasive carcinoma has not been accepted for reference to vulvar carcinoma and is not a recommended term. Solitary stage IA tumors with a depth of invasion of 1 mm or less have essentially no risk of regional node metastasis, and node sampling or resection is not contributory in these.
Histopathology of stage IA vulvar squamous cell carcinoma
The FIGO staging of vulvar carcinoma has defined a stage IA subset of stage I carcinoma as originally set forth by the ISSVD, as a tumor with a diameter of 2 cm (20 mm) or less with a depth of invasion of 1 mm or less (Figure 10). Measurement of tumor diameter alone is insufficient to define the risk of nodal metastasis. Most vulvar carcinomas with a depth of invasion of 1 mm or less are less than 2 cm in diameter. Clinically, VIN surrounding the tumor may be included erroneously in the measurement of the tumor diameter. In superficially invasive vulvar carcinoma, it is essential that the entire tumor be available for study before an attempt is made to determine the depth of invasion. Cases in which there is more than one tumor are not included in the stage IA group. The depth of invasion is defined as the measurement from the epithelial–dermal junction of the adjacent most superficial dermal papilla to the deepest point of invasion, as described by Wilkinson and colleagues.41, 49, 52 The ISGP and WHO have accepted this definition of the “depth of invasion” in the vulva for the purposes of staging a case as stage IA and the tumor does not invade beyond 1 mm.4, 41 This measurement should be distinguished from the “thickness of the tumor”, which is defined as the measurement from the surface, or bottom of the granular layer of keratin, if present, to the deepest point of invasion (Figure 11). The ISGP and WHO recommend that the pathologist report both measurements whenever possible. The stage IA group includes patients whose tumors involve capillary-like spaces, provided skin measurements are available. A protocol for the evaluation of vulvar specimens drafted by the author has been published by the College of American Pathologists.37
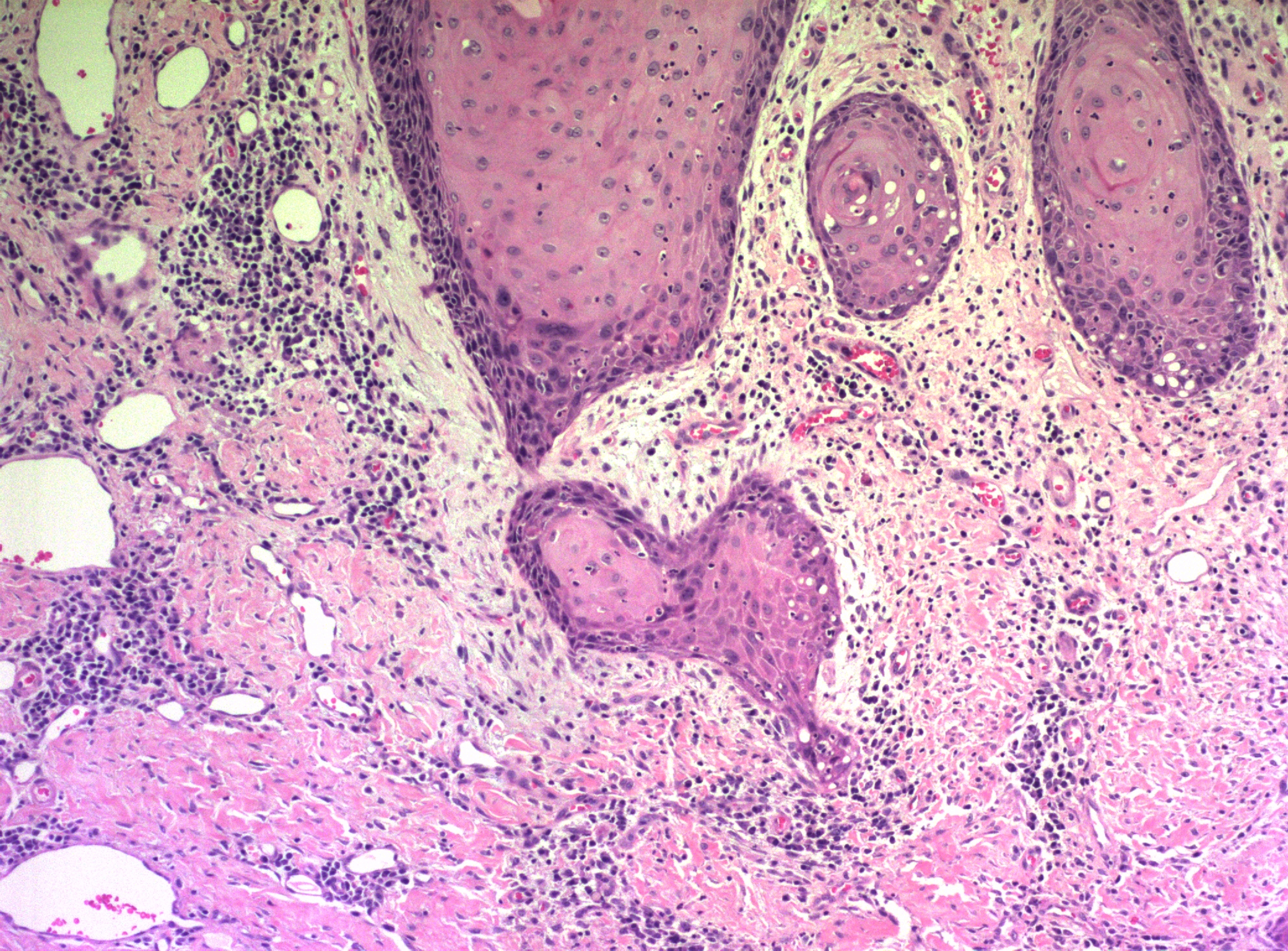 Figure 10 Early superficial invasion in squamous cell carcinoma is evidenced by the irregular shape of the nest of cells at the lower end of the field, and by the accumulation of more abundant eosinophilic cytoplasm in the tumor cells
Figure 10 Early superficial invasion in squamous cell carcinoma is evidenced by the irregular shape of the nest of cells at the lower end of the field, and by the accumulation of more abundant eosinophilic cytoplasm in the tumor cells
Figure 11 Measurement methods of squamous cell carcinoma of the vulva. Depth of invasion is defined as the distance from the epithelial–stromal junction of the adjacent most-superficial dermal papilla to the deepest point of invasion. Tumor thickness is defined as the distance from the surface (mucosa) or bottom of the granular layer of the keratin (skin) to the deepest point of invasion.
Treatment
Stage IA carcinomas, as defined in Table 7, can be treated by wide and deep local excision without lymphadenectomy. In excising a squamous carcinoma, the entire tumor should be included in the excision so that accurate measurements of the diameter, depth of invasion, and thickness of tumor can be assessed. An excision with margins of approximately 2 cm, extending to the fascia (partial deep vulvectomy), is recommended.36 If the tumor is then found to be deeper than 1 mm (no longer a stage IA) on pathologic examination, ipsilateral lymphadenectomy is indicated unless the tumor is midline, in which case bilateral inguinal–femoral lymphadenectomy is necessary. Total or more complete partial deep vulvectomy may be necessary if the tumor extends to a margin of resection or is more than 3 mm deep.
Table 7. Suggestions for sampling tissue removed for diagnosis or treatment of vulvar cancer
Representative sections to include if appropriate:
— Site of deepest invasion
— Interface of tumor with adjacent epithelium
Resection margins
Sections of abnormal epithelium or other tissue remote from tumor
Sections of area(s) marked by surgeon
Sections of prior biopsy or resection site of tumor if no tumor present grossly
Clinical follow-up
Superficially invasive vulvar squamous cell carcinomas that meet the definition of stage IA have essentially no risk of nodal metastases. In contrast, with tumors 3 mm deep, the risk of nodal metastasis is approximately 12%. With a 5 mm depth of invasion, or thickness, there is a reported risk of nodal metastases of approximately 15%.36
Follow-up of women with vulvar superficially invasive carcinoma is essential because it is recognized that these women, although they have a relatively low risk of local recurrence, are at risk of having a “reoccurrence” of a new primary tumor or the vulva, independent of the original tumor site. Although this risk is low, awareness and observation, with biopsy when indicated, can reduce the risk of metastases or death from a recurrent or new “reoccurrent” vulvar carcinoma.36, 51
FRANKLY INVASIVE SQUAMOUS CELL CARCINOMA OF THE VULVA
Frankly invasive squamous cell carcinomas include all of those carcinomas that invade to a depth beyond the limit used to define superficially invasive carcinoma (1 mm).
Histopathology
Histologic subtypes of squamous cell carcinomas are listed in Table 8. Squamous cell carcinomas with keratin pearls and obvious squamous cellular characteristics are considered well differentiated (Figure 12). This pattern is usually the most frequent histologic type encountered. Other terms that have been suggested for the keratin pearl-forming carcinomas include large cell keratinizing, keratinizing, and epidermoid carcinoma. These tumors are more common in older women and are usually not associated with HPV.15 The less well-differentiated squamous cell carcinomas are less obviously squamous in origin without prominent intercellular bridges or keratinization.
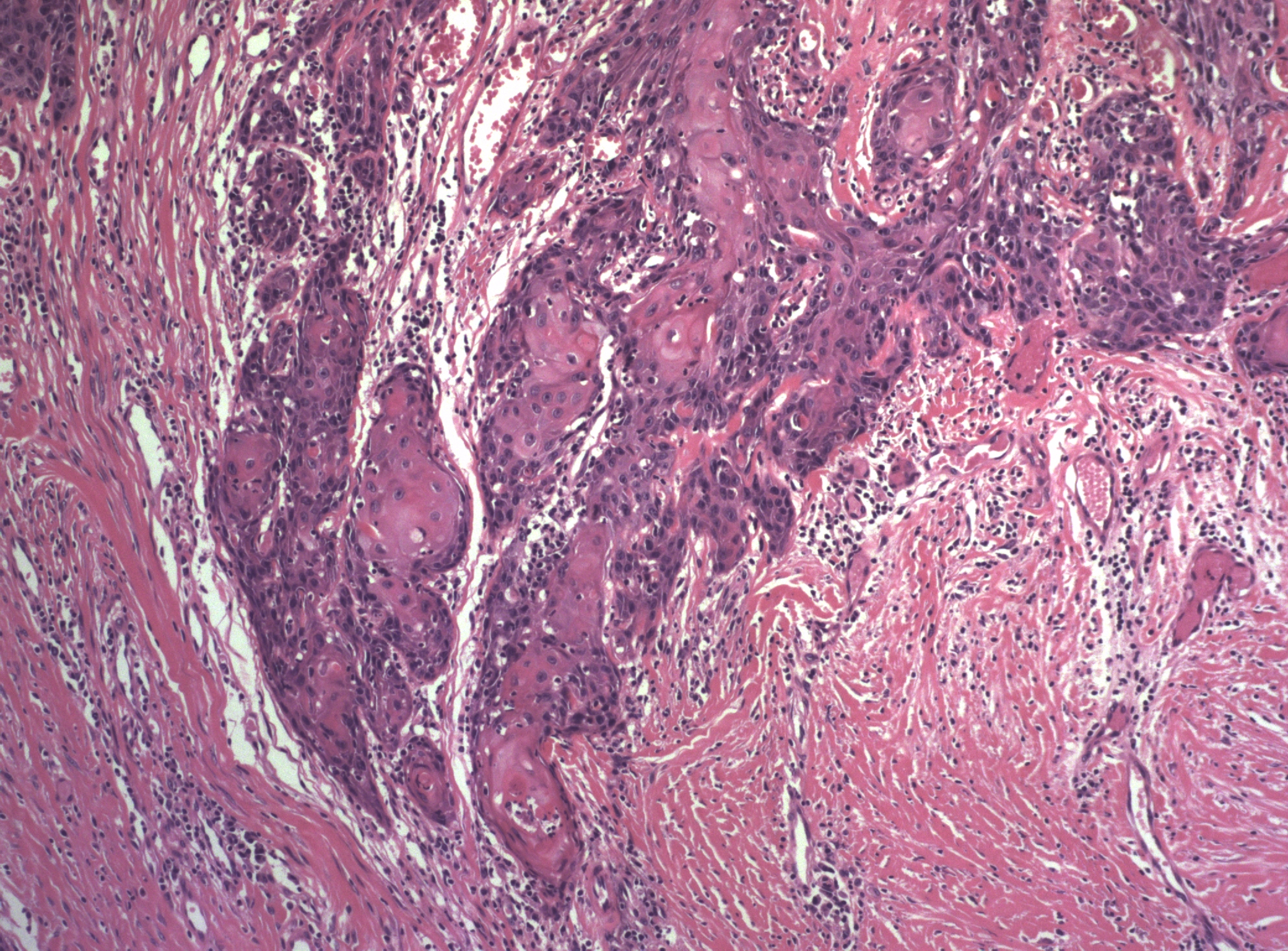 Figure 12 The invasive front of a well-differentiated keratinizing squamous cell carcinoma
Figure 12 The invasive front of a well-differentiated keratinizing squamous cell carcinoma
Table 8. Histologic subtypes of vulvar squamous cell carcinoma including basal cell carcinomas
| Squamous cell carcinoma, well-differentiated (not otherwise specified) |
| Basaloid carcinoma |
| Warty (condylomatous) carcinoma |
| Verrucous carcinoma |
| Giant cell squamous carcinoma |
| Spindle cell squamous carcinoma |
| Acantholytic squamous cell carcinoma (adenoid squamous carcinoma) |
| Lymphoepithelioma-like carcinoma |
| Basal cell carcinoma |
| Metatypical basal cell carcinoma (basosquamous carcinoma) |
| Adenoid basal cell carcinoma |
| Sebaceous cell carcinoma |
From Wilkinson EJ: Premalignant and malignant tumors of the vulva. In Kurman RJ: Blaustein's Pathology of the Female Genital Tract, 5th ed. New York, Springer-Verlag, 2001.
Basaloid carcinomas are described as squamous tumors that do not form keratin and are composed of smaller cells with an increased nuclear-to-cytoplasmic ratio (Figure 13). These are less common than the well differentiated tumors and are associated with HPV, especially type 16.15
 Figure 13 Basaloid squamous cell carcinoma. The cells have scant cytoplasm, but there is still evidence of squamous differentiation towards the center of the cell nests
Figure 13 Basaloid squamous cell carcinoma. The cells have scant cytoplasm, but there is still evidence of squamous differentiation towards the center of the cell nests
Warty (condylomatous) carcinomas have superficial features of condyloma acuminatum. Their prognosis appears to be intermediate between well differentiated squamous cell carcinomas and verrucous carcinomas. They also are associated with HPV, especially type 16.15
The giant cell forms of vulvar carcinoma are characterized by multinucleated tumor giant cells and may have a poorer prognosis than the well-differentiated carcinoma. These tumors must be distinguished from malignant melanoma, which also may have tumor giant cells. An analysis of cases classified as giant cell carcinoma of the vulva showed that criteria described for giant cell carcinoma may be observed in vulvar malignant melanoma and that the use of immunohistochemical studies is essential to distinguish between the two (see section on melanoma, below).42
In a study of 50 squamous cell carcinomas, Lasser and coworkers45 described two patients in whom the tumor showed primarily an adenoid-squamous cell pattern. In 15 other patients, loci of adenoid-squamous change were evident in isolated areas. The adenoid-squamous pattern of growth is characterized by the formation of pseudoglandular spaces lined with a single layer of squamous cells. Within the spaces, dyskeratotic and acantholytic cells may be present. Mucin stains do not show evidence of secretion. These adenosquamous areas are usually found in tumors that would otherwise be considered well-differentiated carcinomas with keratin pearl formation, and this pattern of growth has not been observed in metastases or in recurrent tumors. However, neoplasms with a predominantly adenoid pattern are poorly differentiated and have a poor prognosis. One study noted a 6% 5-year survival for such patients compared with a 77% survival for patients with conventional squamous cell carcinoma.53
Other types of vulvar carcinoma
Other primary carcinomas that have been reported arising within the vulva include verrucous carcinoma, basal cell carcinoma, small cell,54 Merkel cell carcinoma,55, 56, 57 and adenocarcinoma.58 The ISGP has recommended that pathologists report the following information for partial or total vulvectomy specimens.41
1. Depth of tumor invasion (in millimeters)
2. Thickness of tumor (in millimeters)
3. Whether there is vascular space involvement by tumor
4. Diameter of the tumor (in millimeters) measured in the fresh or fixed state
5. Clinical measurement of the diameter of the tumor.
Treatment
The treatment for vulva squamous cell carcinoma is directed toward sparing as much of the vulva as possible with wide local excision (partial deep vulvectomy) and associated ipsilateral regional lymph node dissection (inguinal–femoral lymphadenectomy). A recent study has shown that limited lymphadenectomy may be adequate in assessing the lymph node status in patients with vulvar squamous carcinoma.59 If the tumor is midline or extends near the midline, bilateral inguinal–femoral lymphadenectomy is usually performed.60 If the superficial lymph nodes are involved with tumor, radiation of the deep pelvic nodes is usually recommended.59
VERRUCOUS CARCINOMA
Verrucous carcinomas are an uncommon, special type of tumor with characteristic gross and microscopic features.61 The precursors of verrucous carcinoma are not well understood. Some appear to be intimately associated with condyloma acuminatum, and the so called “giant condyloma of Buschke-Lowenstein” is considered verrucous carcinoma.41 A precursor, described as “vulvar acanthosis with altered differentiation” is also considered a precursor, based on changes found adjacent to verrucous carcinomas.62 Clinically, they are slow-growing, papillary, cauliflower-like lesions that eventually may become necrotic, ulcerated, and fungating.41, 60, They may be locally destructive, and when surgically excised, they commonly recur but rarely metastasize. On histologic examination, verrucous carcinomas show a wart-like character similar to condylomata acuminata, demonstrating numerous branching papillary stalks composed of extremely well differentiated squamous epithelium (Figure 14). Verrucous carcinomas are characterized by a “pushing” border with a well-defined tumor–dermal interface, without apparent infiltration.60 Cellular atypia is minimal and always restricted to the basal layer.60 Atypical mitotic figures are not identified and dyskeratosis and koilocytosis are absent. A chronic inflammatory cell infiltrate within the dermis is usually present. Verrucous carcinomas have been found to be associated with HPV, types 6 and 11 being the most common.61 These tumors are typically DNA diploid.
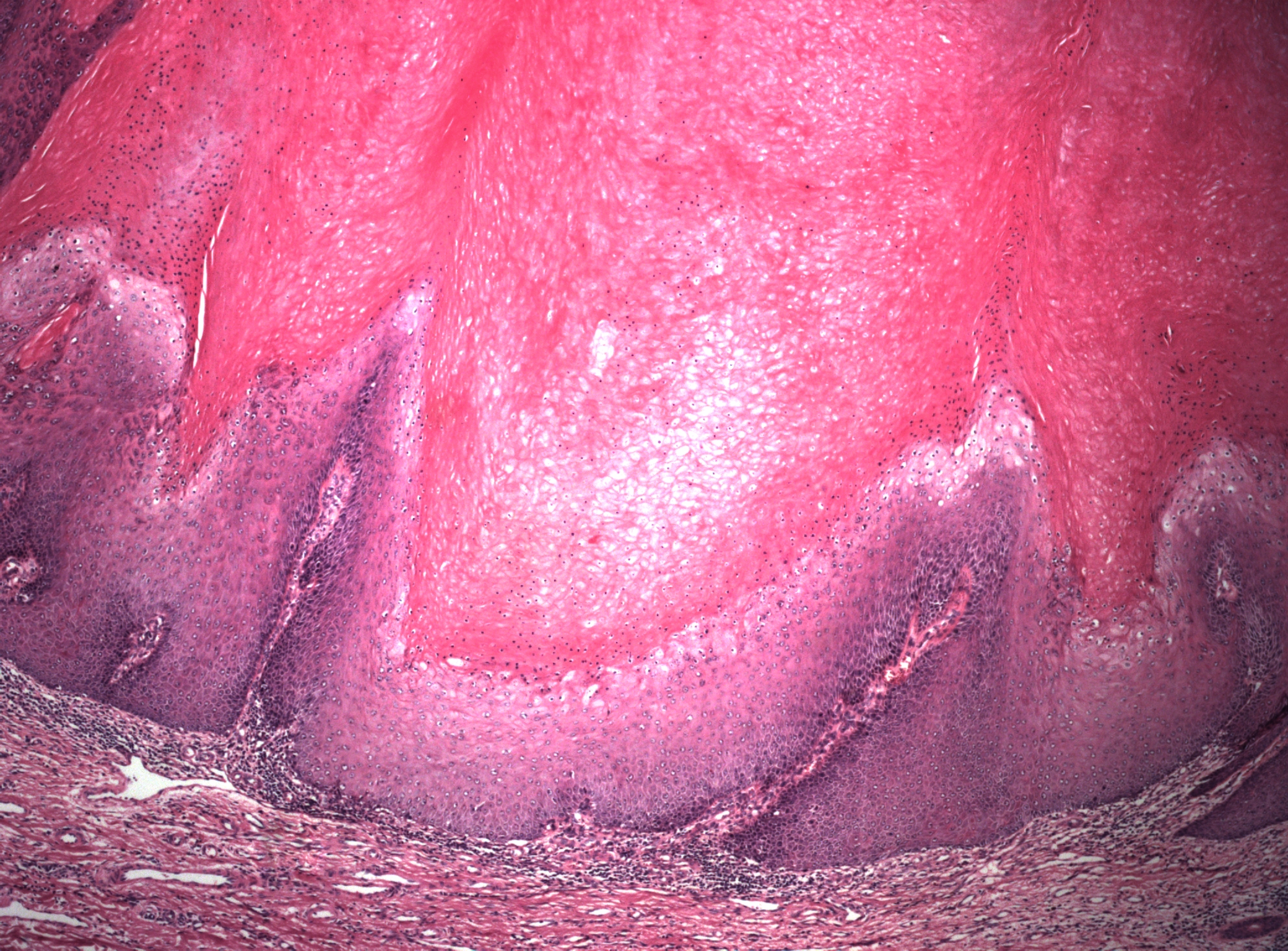 Figure 14 Verrucous carcinoma, showing pronounced hyperkeratosis and the characteristic "pushing" border
Figure 14 Verrucous carcinoma, showing pronounced hyperkeratosis and the characteristic "pushing" border
The main differential diagnoses for verrucous carcinomas include condyloma acuminatum and squamous carcinoma with verrucous features (warty carcinoma). Condylomata are typically not infiltrative, have prominent vascular dermal papillae separating the acanthotic epithelial elements, and have koilocytosis and dyskeratosis. Occasionally, VIN may occur within a condyloma, which can be distinguished by the nuclear pleomorphism and hyperchromasia, abnormal mitoses, and junction with the typical condyloma acuminatum. Warty squamous carcinomas have infiltrating tumors at the tumor–dermal interface, rather than the pushing margins of verrucous carcinoma. Warty carcinomas usually have adjacent warty VIN, with koilocytosis, dyskeratosis, multinucleation, nuclear pleomorphism, and hyperchromasia. Usually abnormal mitotic figures are evident unlike verrucous carcinoma.
Verrucous carcinoma is a slowly growing tumor, but left untreated, it can be deeply infiltrative and locally destructive. The treatment of verrucous carcinoma is partial deep vulvectomy (wide local excision to the fascia). Lymphadenectomy is not necessary if the lymph nodes are not clinically suspicious. If they are suspicious, lymph node biopsy or fine-needle aspiration is recommended to exclude metastasis rather than lymphadenectomy since metastases to regional lymph nodes are not generally encountered.60 In the majority of cases, lymphadenopathy, if present, is related to an inflammatory reaction evoked by the tumor or secondary to infection. Recurrence is uncommon; however, any new lesions require re-excision. Radiation therapy should be contraindicated because of the possibility that the tumor can undergo anaplastic change with subsequent aggressive behavior after radiation.60 Local application of podophyllin, bleomycin therapy, and cryosurgery are considered ineffective methods of treatment.63 Recently, there has been a report of control of tumor progression using acitretin in a case of verrucous carcinoma not amenable to surgery.64
BASAL CELL CARCINOMA
Basal cell carcinomas constitute 2–3% of all vulvar malignancies, but make up approximately 65% of all nonvulvar cutaneous malignancies. Vulvar basal cell carcinoma can be found in women younger than 30 years of age, but is most commonly encountered in older women.65, 66, 67
The clinical presentation of basal cell carcinoma has been divided into three basic types. The first type is the nodulo-ulcerative lesion, which may appear as a deep ulcer with raised margins. The second presentation is a more superficial, flat lesion with a waxy surface that is macular and slightly erythematous. The third type appears as a polypoid excrescence with an intact surface. These three clinical patterns may occur in any combination and carry no particular significance with regard to prognosis, but rarely is the diagnosis made on clinical appearance alone. The tumor is typically solitary and confined to the vulva, usually involving the labia majora. Multiple basal cell carcinomas can be seen in the autosomal-dominant nevoid basal cell carcinoma (of Gorlin) syndrome and may be associated with other anomalies, including jaw cysts and skeletal abnormalities.68 The finding of a basal cell carcinoma should prompt a search for basal cell lesions elsewhere on the skin, although the majority of basal cell carcinomas are not syndrome-associated. In a small study of basal cell carcinoma of the genitalia, no predisposing risk factor or evidence of HPV infection was identified.69
Microscopic features and behavior of basal cell carcinomas of the vulva are the same as those of basal cell carcinomas elsewhere in the skin. Basal cell carcinoma derives its name from the similarity of the tumor cells to the normal basal cells of the epidermis and it is composed of relatively uniform cells with prominent palisading without keratinization. The tumor usually has a well-defined infiltrating margin. A desmoplastic reaction is common in the stroma (Figure 15). Keratinization may be noted with a basal cell carcinoma, but this does not suggest a transformation to squamous carcinoma. Such tumors are termed metatypical basal cell carcinomas. Pigmented basal cell carcinomas have pigment in the dermal macrophages rather than within the tumor cells themselves. These variations do not imply a difference in the prognosis of the lesion.
.jpg) Figure 15 Basal cell carcinoma of the vulva. The appearance is identical to that of these tumors elsewhere in the skin, with peripheral palisading of the small cells with scant cytoplasm and a desmoplastic stroma
Figure 15 Basal cell carcinoma of the vulva. The appearance is identical to that of these tumors elsewhere in the skin, with peripheral palisading of the small cells with scant cytoplasm and a desmoplastic stroma
A distinction must be made between basal cell carcinoma and adenoid cystic carcinoma of the vestibular glands of the vulva. Adenoid cystic carcinoma of the vestibular glands is very rare and characterized by cribriform gland-like spaces filled with eosinophilic material. This distinction is necessary because, unlike the basal cell carcinoma, the adenoid cystic carcinoma is an aggressive lesion prone to producing distant metastases.70 Basal cell carcinoma expresses Ber EP4 that can be identified by immunohistochemical study and is of value in distinguishing it from similar appearing tumors, including VIN.71
The treatment of choice for basal cell carcinoma is local excision.72 Vulvectomy is not indicated nor is removal of nodes necessary. The frequency of nodal metastasis in vulvar basal cell carcinoma is extremely low and is largely restricted to the deeply invasive lesions.73 Local recurrence on the vulva has been reported and, therefore, long-term follow-up is important.74
MELANOMA
Melanoma (malignant melanoma) is the second most common malignant tumor of the vulva, but it is much less frequent than squamous cell carcinoma. Melanoma accounts for approximately 9% of all malignant neoplasms of the vulva. Patients with melanoma of the vulva reportedly vary in age from 17 to 80 years of age and older (mean 55 years). Such tumors may arise either in a pre-existing pigmented nevus or from apparently normal skin. They should be distinguished from benign nevomelanocytic nevi and atypical or dysplastic nevi. Consultation with an expert dermatopathologist with skill in this area is essential in questionable cases. Approximately one half of the cases of vulvar melanoma involve mucosal sites, whereas the rest involve the skin. Melanomas are relatively rare in persons with heavily pigmented skin. They are unrelated to parity. The most common presenting complaint is bleeding or a lump noted by the patient.75, 76 Additional symptoms include pruritus and change in color, shape, or size in a pre-existing pigmented lesion.74 Melanomas clinically appear pigmented in approximately 70% of cases; approximately 30% are macroscopically amelanotic.74 Malignant melanomas of the vulva occur with nearly equal frequency on the clitoris, labia minora, or labia majora and are typically solitary lesions.74 The microscopic appearance is similar to that of melanoma of the skin.
The histopathologic types of melanoma include nodular melanoma, superficial spreading melanoma, and acral lentiginous melanoma.77, 78 Nodular and superficial spreading melanomas tend to predominate in most series, with acral lentiginous tumors comprising less than one fifth of the cases. Variations in histopathologic criteria to separate nodular from superficial spreading melanoma may account for some of the observed variations in reported frequency. Nodular melanomas have a predominantly vertical growth pattern, involving fewer than four rete ridges adjacent to the tumor. If the radial growth of the adjacent superficial component of the melanoma involves four or more adjacent rete ridges, the tumor can be classified as a superficial spreading melanoma regardless of the shape or nodularity of the vertical, or invasive, component of the tumor.79
Cell types of melanomas include epithelioid, spindle, nevoid (dendritic), and mixed varieties (Figure 16). With lesions of the vulva, there is no significant correlation between survival rates and cell type, the presence or absence of pigment, or epithelial or inflammatory cell reaction. The prognosis for patients with primary melanoma without metasasis depends on the thickness of the tumor and the depth of invasion. In determining the level of invasion of a melanoma, Clark and coworkers divided melanomas into five groups.80 Clark's levels are readily applied to vulvar skin, but may have some limitations in mucosal sites.81
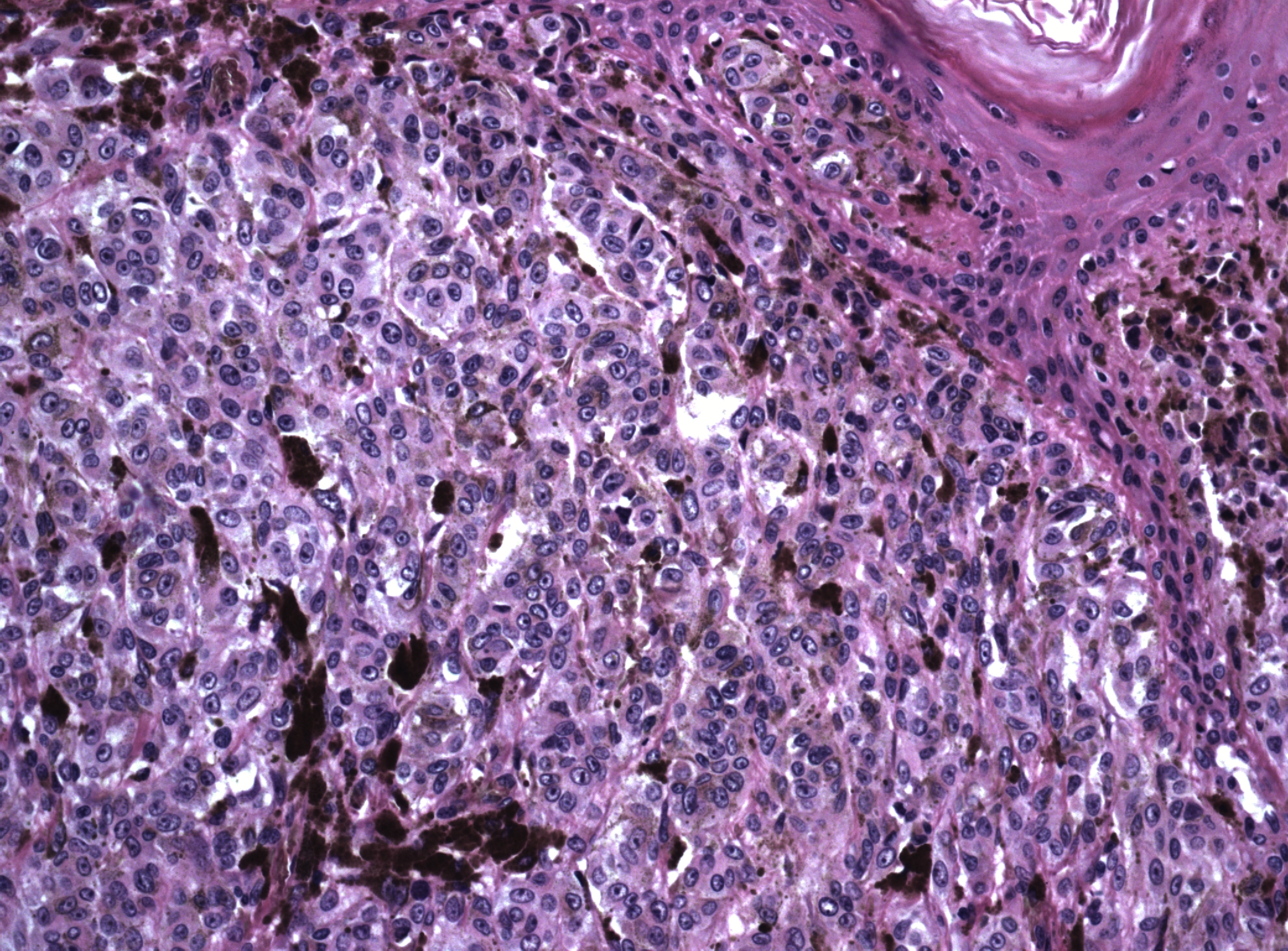 Figure 16 Malignant melanoma of vulva. A focus of uninvolved overlying epidermis is present in the upper right. The dermis is filled with large cells with round nuclei, vesicular chromatin and prominent nucleoli. Many of the tumor cells contain dark brown melanin pigment
Figure 16 Malignant melanoma of vulva. A focus of uninvolved overlying epidermis is present in the upper right. The dermis is filled with large cells with round nuclei, vesicular chromatin and prominent nucleoli. Many of the tumor cells contain dark brown melanin pigment
Level I: Lesion confined to the epidermis (in situ). These have no measurable thickness.
Level II: Lesion penetrates the papillary dermis below the basement membrane. These are usually 0.75 mm or less in thickness.
Level III: Tumor cells aggregate at the junction of the papillary and reticular dermis. These are more than 1 mm but not more than 2 mm in thickness.
Level IV: Tumor cells infiltrate the reticular dermis. These exceed 2 mm in thickness, but do not invade fat or deeper dermal structures.
Level V: Tumor invades the underlying fat.
Breslow82 showed that the depth of invasion measured in millimeters from the top of the granular layer, or its estimated position in the epidermis, correlated well with the levels of Clark and associates80 and was a more readily learned technique. In addition, excellent precision was noted when pathologists compared measurements. In melanomas that involved the nonkeratinized vulvar mucous membrane, the most superficial layer of squamous cells corresponded to the granular layer. When this leveling system was used, nodular melanomas were found to have deeper penetration than the other types, a fact that most probably accounted for their poorer prognosis. It was concluded that both the thickness and the level of invasion have an impact on prognosis.
Prognosis and treatment
The prognosis for patients with vulvar melanoma is worse than that for patients with cutaneous melanoma from most other sites.74, 75 The number of positive lymph nodes seems to represent the strongest prognostic factor in melanoma of the vulva.83 Level of thickness of the primary tumor is also an important prognostic parameter.84 The prognosis of vulvar melanoma is excellent with thin tumors at Clark level II or less or with a thickness measuring 1.49 mm or less.85 Most melanomas of the vulva are advanced (Clark's level III or IV) at the time of diagnosis.86 The usual treatment for vulvar melanomas with a thickness of 0.75 mm or less, and in most cases for 1 mm or less is wide local excision with a 1.0-cm circumferential and 1.0–2.0-cm-deep margin. Melanomas with a thickness of 1–4 mm thick require 2.0-cm surgical margins with deep margins of at least 1.0–2.0 cm.87 Melanomas with a thickness greater than 4 mm are usually treated by wide excision to the fascia, or partial or total vulvectomy.88 Depending on the tumor size, the surgical procedure may include bilateral inguinal lymphadenectomy.89, 90 Total deep vulvectomy with bilateral inguinal–femoral lymphadenectomy (radical vulvectomy does not appear to improve survival when compared with deep local excision with bilateral groin lymphadenectomy). Lymphatic mapping, to sample the sentinel lymph node, is currently being used in some centers. The sentinel node is identified using peritumoral injection of a blue dye or technetium 99 (Tc99) colloid or both and identification of the primary draining nodes. These are evaluated. If tumor is present, total lymphadenectomy is done; if the sentinel lymph node or nodes are negative, no further node dissection is done.91, 92
Recurrence of melanoma may be local or metastatic. Prognosis after recurrence is poor, with a reported 5-year survival of 5%.76 Metastatic sites from the vulva include the regional lymph nodes as well as the vagina, cervix, bladder, urethra, and rectum.5, 6, 9, Lymphatic and vascular spread to the lungs, brain, and bone marrow is well documented.93
Cutaneous melanomas tend to spread initially via lymphatics, and metastases are usually first noted in the node groups draining the tumor site. Thus, metastatic disease from the vulva usually involves the groin nodes; however, metastases can occur in almost any organ as a result of hematogenous spread. Early detection is, therefore, particularly important. Because it is not possible to differentiate all dark lesions of the vulva on clinical examination alone, all elevated pigmented lesions 4 mm or larger in diameter on the vulva should be excised. Although the majority of such lesions will prove to be vulvar melanosis, lentigines, nevi of various types, seborrheic keratosis, pigmented condyloma acuminatum, or VIN, these lesions are sometimes clinically indistinguishable from early superficial spreading melanoma.94 If a lesion is suspected of being a melanoma on the basis of clinical appearance, the optimal approach is to excise the lesion locally, widely, and deeply, providing generous margins. It is necessary for the physician to think in three dimensions when approaching such lesions and to appreciate the fact that the tumor may well be as deep as it is wide. The value of this type of approach (compared with partial excision, or shave biopsy) is twofold:
1. To establish the level of the melanoma, the entire depth of the lesion must be studied. Local biopsy will not allow assessment of the depth or level of infiltration. In fact, a superficial biopsy is not adequate for this purpose because the lesions may be misinterpreted as active junctional nevi or atypical nevi misinterpreted as melanoma.
2. The deep excisional biopsy may serve as the definitive treatment for the patient. With this approach, the problem of attempting to excise residual tumor buried by a prior biopsy, necessitating excessive surgery, can be avoided.
The differential diagnosis of melanoma is extensive and includes typical junctional and congenital nevi, atypical vulvar nevi, squamous cell carcinoma, extramammary Paget disease, and soft tissue tumors.95 Immunohistochemistry can be very useful in distinguishing melanomas from nonmelanocytic tumors in that melanomas are usually immunoreactive for S-100 and melanoma-specific antigen (HMB-45), Melan-A, and other melanoma markers but nonimmunoreactive for cytokeratin.26, 41, 96
BARTHOLIN GLAND TUMORS
Bartholin gland malignancy is a relatively rare lesion, accounting for approximately 1–2% of all carcinomas of the vulva.22, 69, 97, 98, Bartholin gland malignancy is usually a disease of the older age groups, and primary tumors generally present as enlargement of the Bartholin gland. However, because of common benign causes of Bartholin gland enlargement, this finding initially may be misinterpreted clinically as a cyst or abscess. Therefore, any bleeding from a Bartholin duct or a persistent nodule in the Bartholin gland area in women older than 50 years warrants excision of the entire gland. Even in younger women, a Bartholin mass that persists after marsupialization of a Bartholin cyst requires histologic evaluation if no other explanation is evident to rule out malignancy.
Criteria for establishing a tumor as a primary Bartholin gland carcinoma include that the tumor (1) is histologically consistent with a Bartholin gland carcinoma; (2) is connected to the Bartholin gland duct; (3) contains a transitional area from benign to malignant in the gland; and (4) is not metastatic, as recognized by histologic features consistent with a known primary. In many cases, these criteria cannot be fulfilled, usually because of the inability to define any normal adjacent Bartholin gland tissue.
The histologic type of Bartholin gland carcinoma reflects the origin of the tumor. The Bartholin gland and its duct are composed of three types of epithelium: the secretory cells of the gland acini, transitional cells lining the duct, and squamous cells lining the vestibular orifice. In the glandular or acinar secretory portions of the gland, adenocarcinomas arise, accounting for approximately 40% of the cases. Squamous cell carcinomas account for another 40% and may arise from squamous metaplasia of the ductal epithelium. Such squamous carcinomas are generally nonkeratinizing, and a pattern of basal cell type may be seen (Figure 17). Adenoid cystic carcinomas comprise approximately 15% of the cases, with transitional cell carcinoma, adenosquamous carcinoma, and undifferentiated carcinomas each comprising 5% or less of the total.22, 69, 99, Primary small cell neuroendocrine carcinoma of the Bartholin gland has been reported. Bartholin gland adenocarcinomas, adenosquamous carcinomas, and squamous cell carcinomas are usually immunoreactive for carcinoembryonic antigen. The adenocarcinoma and adenosquamous carcinomas typically contain mucin.52 Adenoid cystic carcinomas are characterized by their acinic architecture, in which acellular eosinophilic material is seen within the glandlike acini. This material has been characterized as a basement membrane-like material. These tumors are keratin immunoreactive and contain S-100 antigen, which may reflect a myoepithelial component. Occasionally, mixed tumors that show both squamous and adenomatous elements are found. The prognosis is probably not influenced by cell type but rather by the presence or absence of lymph node involvement. Lymphatic spread to the superficial groin nodes and deep pelvic nodes is common, and early nodal involvement most probably accounts for the relatively poor (30%) 5-year survival rate.
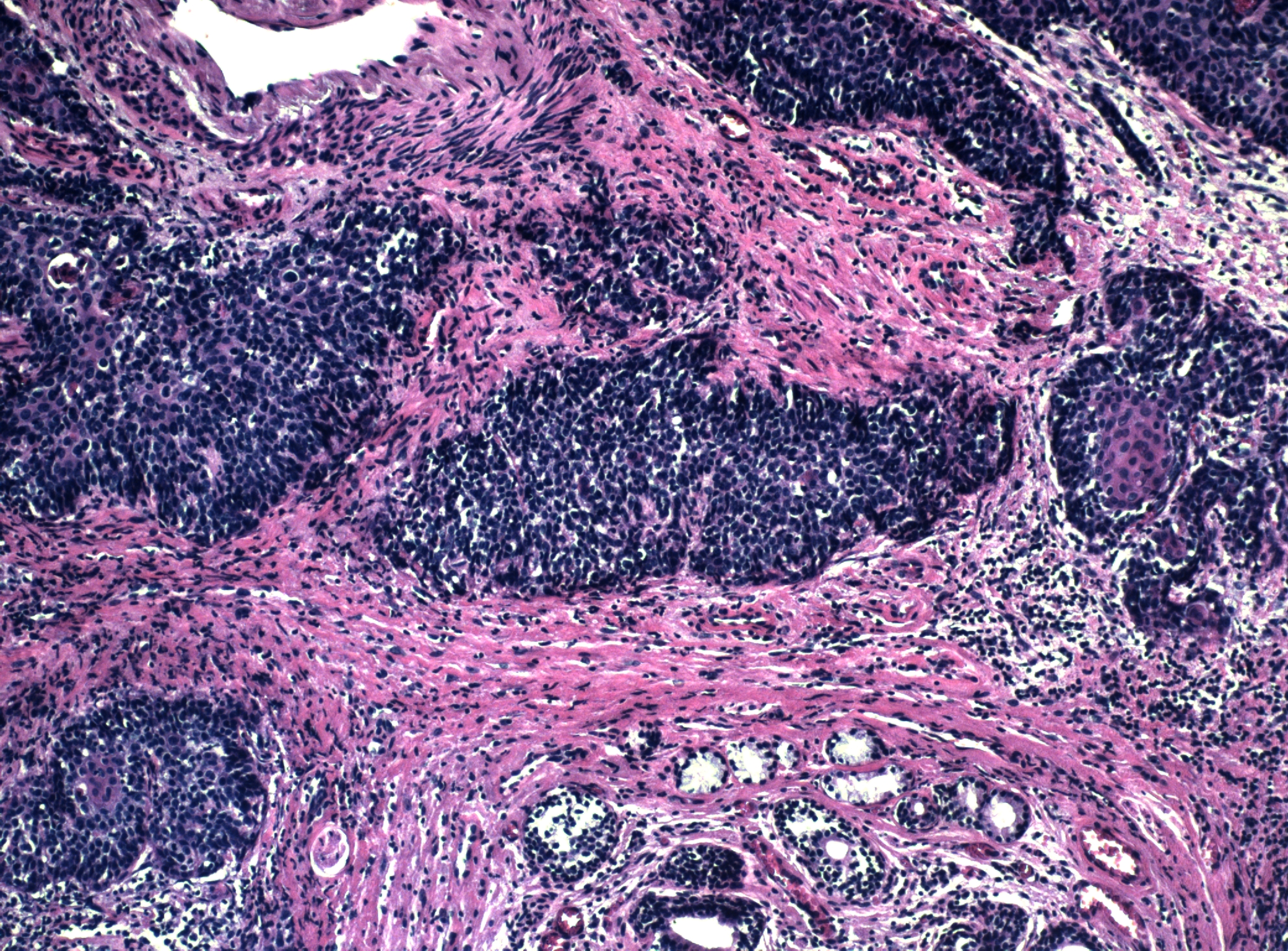 Figure 17 Bartholin gland carcinoma. Residual glandular epithelium can be seen at the bottom of the field. The tumor has a predominantly basaloid morphology, but focal squamous differentiation can be seen in the center of the nest of tumor cells on the right
Figure 17 Bartholin gland carcinoma. Residual glandular epithelium can be seen at the bottom of the field. The tumor has a predominantly basaloid morphology, but focal squamous differentiation can be seen in the center of the nest of tumor cells on the right
Bartholin gland adenocarcinomas must be distinguished from hidradenomas, which are benign primary neoplasms of the skin appendages. Hidradenomas may exhibit bizarre glandular architecture on low-power microscopy, but the component cells are arranged in a typical epithelial and myoepithelial cell fashion and lack the characteristics of malignancy. Complete excision shows the well-defined relationship of this benign tumor within the underlying dermis.
Bartholin gland carcinomas occur deep beneath the inferior labia minora where there is access to lymphatics. The surgical treatment is wide and deep partial vulvectomy with ipsilateral inguinal-femoral lymphadenectomy. If the inguinal lymph nodes are involved with tumor, pelvic radiation therapy is the usual treatment.
The prognosis correlates with the involvement of the inguinal lymph nodes by metastatic tumor. In approximately one fifth of the cases, the inguinal nodes are involved at the time of diagnosis. When this occurs, the 5-year survival rate is less than 20%. When the nodes are not involved, the survival is approximately 50% at 5 years. Unlike adenocarcinoma or squamous carcinoma of Bartholin gland, adenoid cystic carcinomas have a predilection for local recurrence and only infrequently metastasize.
Although many adenocarcinomas of the vulva are of Bartholin's gland origin, primary adenocarcinomas arising from cloacal remnants, Paget disease, skin appendages, vulvar mammary-like glands, and Skene glands have been reported.57, 100, 101, 102, Tumors, including undifferentiated sweat gland adenocarcinoma, eccrine porocarcinoma, hidradenocarcinoma,100 and ductal eccrine carcinoma,100 as well as clear cell hidradenocarcinoma, apocrine carcinoma, and variants of adenocarcinoma, have all been reported.
SOFT TISSUE MALIGNANCY
Soft tissue malignancies involving the vulva include a broad range of lesions of diverse origin. Although the superficial appearance of such lesions may be unremarkable, observations regarding fixation of the tumor to the overlying epidermis and the presence or absence of fixation to the deeper fascia may prove most helpful in later diagnostic analysis.
Sarcoma of the vulva is relatively rare and accounts for 1.1–3% of all vulvar malignancies. Sarcomas may occur in any age group but are seen most frequently among women between the ages of 30 and 50 years. Most sarcomas arise from the labia majora.26
Leiomyosarcoma is the most common type encountered in the vulva.26 These tumors are usually not ulcerated and appear as a firm nodule in the deeper subcutaneous tissues, possibly fixed to the underlying fascia. When the lesions are large, an incisional biopsy can be performed; when the lesions are small, total excision is preferred. Microscopically, leiomyosarcomas are characterized by interlacing bundles of smooth muscle cells with anaplastic-appearing nuclei and numerous mitotic figures (Fig. 18). The criterion used to distinguish leiomyosarcoma from leiomyoma is a mitotic count of at least ten per 10 high-power fields (HPF) or evidence of invasion or metastasis, or both.103 Necrosis is a common and important finding in leiomyosarcoma. There is an indeterminate group of smooth muscle tumors of the vulva that have an uncertain risk of recurrence. They have infiltrative margins and a mitotic rate between 5 and 9/10 HPF without significant nuclear atypia. If three or more of the following four criteria are fulfilled, the lesion is considered a leiomyosarcoma: significant nuclear atypia is present, the mitotic rate is 5/10 HPF or higher, the greatest tumor diameter is 5.0 cm or more, and the margin is infiltrative.102 Tumors with only one of these features are considered leiomyomas and tumors with only two of these features may be classified as atypical leiomyomas, which are still considered benign or indeterminate. Because the indeterminate and malignant smooth muscle tumors infiltrate the adjacent fat and fascia, wide margins should be included in the excision. Leiomyosarcomas may carry a good prognosis if adequately and completely excised. Wide and deep local excision, without regional lymphadenectomy, is the preferred approach for a leiomyosarcoma confined to the vulva. If metastasis occurs, it may be remote, with pulmonary or hepatic involvement common.
 Figure 18 Leiomyosarcoma of vulva. The tumor is composed of spindled cells with marked nuclear pleomorphism and atypia.
Figure 18 Leiomyosarcoma of vulva. The tumor is composed of spindled cells with marked nuclear pleomorphism and atypia.
Fibrous histiocytomas can be malignant or benign. Those confined to the dermis, without infiltration into fat or subcutaneous fascia, are generally considered benign lesions, although they have the potential to recur locally. Wide local excision with an adequate deep margin is the treatment of choice. Careful pathologic examination of the deep margins is mandatory because lesions that involve the underlying fat, fascia, or muscle must be considered malignant fibrous histiocytomas (MFHs) until proved otherwise. MFH is rare on the vulva, but it is the second most common sarcoma of this site. This tumor typically presents as a solitary deep subcutaneous nodule or an ulcerated mass in middle-aged women. It is an aggressive tumor that freely infiltrates subcutaneous fat, fascia, and muscle.104 The tumor is thought to arise from histocytes that have undergone fibroblastic differentiation. MFH is characterized by marked pleomorphism with giant and multinucleated cells. The treatment is wide local excision or radical vulvectomy. Lymphadenectomy is reserved for those cases with clinical evidence of regional node involvement. Postoperative radiation therapy is believed to be of value in reducing local recurrence. The prognosis in patients with primary MFH is guarded.103
Dermatofibrosarcoma protuberans (DFSP) occurs predominantly in postmenopausal with a median age of 54 years, however, it may occur in younger women, and in pregnancy has been recognized to exhibit rapid growth. At presentation the tumor typically presents as a superficial firm subcutaneous nodule, commonly exceeding 5 cm in greatest dimension.105 The overlying skin may have increased pigmentation. These tumors may infiltrate subcutaneous fat, but usually do not infiltrate into underlying fascia. Satellite tumor nodules peripheral to the primary tumor are evidence of more aggressive growth.
Microscopically, these three neoplasms (benign and MFHs and DFSP) show a similarity of cell type and tissue organization. The cells are spindle shaped and often have a foamy cytoplasm. They are usually arranged in haphazard bundles, resembling straw cast on the ground, the so-called storiform pattern.
By immunohistochemical study DFSP expresses CD34, which may be useful in distinguishing it from dermatofibroma that is typically negative. To further distinguish these soft tissue tumors additional immunohistochemical studies are usually needed. These and additional microscopic features are needed to distinguish most soft tissue tumors. The reader is referred to texts in gynecologic pathology and soft tissue tumors to establish these diagnosis.26, 41, 106, 107
DFSP is usually treated by partial deep vulvectomy with the objective of complete excision of the tumor with clear margins. Survival following treatment is reported in over 90% of cases. Local and regional recurrence are recognized and reported in approximately one-fifth to one-half of the cases. Distant lymph node involvement and/or pulmonary metastasis are infrequent.
Rhabdomyosarcomas (RMS) of the vulva are most commonly of botryoid, alveolar, or embryonal type and each of these types have distinctive presentations. Regardless of tumor type rhabdomyosarcoma is an uncommon tumor of the vulva of probable striated muscle origin. In children it is the most common soft tissue tumor overall, and approximately one-fifth of rhabdomyosarcomas in children arise in the genital tract.108, 109 In adults RMS is a rare tumor and the vulva is an infrequent site, with most RMS arising in the cervix or uterus.
There are limited data regarding the frequency of histologic subtypes of RMS of the vulva, however, botryoid RMS, alveolar RMS, and embryonal RMS are the predominant histopathologic types.41, 108 These subtypes are distinguished by microscopy and cytogenetic studies. The histologic type influences prognosis.
Vulvar botryoid rhabdomyosarcoma usually involves mucosal sites of the labia, perineum, and rarely the clitoris presenting as a submucosal or polypoid mass (sarcoma botryoides). Most cases of RMS present before 11 years of age with vulvar botryoid RMS presenting primarily in children less than 2 years of age.110
On microscopic examination, botryoid RMS is usually polypoid and has a characteristic cambium layer, which is a narrow, richly cellular, subepithelial layer of tumor cells beneath a thin epithelial surface separated by an edematous and/or myxoid relatively hypocellular zone. Within the cellular areas round to spindle‑shaped rhabdomyoblasts with prominent eosinophilic cytoplasm and relatively large nuclei can often be identified. These cells have cross striations in some cases, and mitotic figures are usually present.
The tumor cells of most of the rhabdomyosarcoma are usually immunoreactive for muscle specific actin (HHF-35) and desmin, other skeletal markers may also be of value.
Tumor invades locally with late metastasis to regional lymph nodes, lungs, and distant sites. Prognosis is dependent on the clinical stage of the tumor, size of the tumor, and histologic type. Localized tumors under 5 cm in greatest dimension carry an excellent prognosis with treatment. Botryoid tumors have a better prognosis than other types of RMS, with a 5-year survival exceeding 90%. Embryonal RMS without distinguishing features is associated with an intermediate prognosis, whereas patients with alveolar rhabdomyosarcoma may have a more guarded prognosis. Therapy may require conservative surgery if needed, with chemotherapy being the main approach and radiation therapy as an addition if deemed necessary.
Alveolar rhabdomyosarcoma can be a primary vulvar tumor and is reported predominantly in children less than 7 years of age, and may present at birth.111 It is also reported in adults. As a subcutaneous tumor it may present as a mass with red to blue discoloration of the overlying skin, giving the mass a “blue berry muffin” appearance. Clinically the vulvar enlargement is that of a subcutaneous mass that may resemble a Bartholin cyst or perianal abscess.
On microscopic examination the tumor has round to spindle shaped cells with scant cytoplasm with typical rhabdomyoblasts and “strap” cells with cross striations being rare. When present these cells are usually identified in deeper areas. The alveolar pattern is produced by tumor cell lined cystic spaces supported by fibrovascular septa, and hypocellular adjacent dermis. This appearance of the tumor may only be focal. Multinucleated tumor giant cells, with nuclei having a “wreath-like” appearance, may be present.108
Immunohistochemical findings are as described for botryoid rhabdomyosarcoma and are dependent somewhat on the degree of tumor differentiation.
Somewhat over one-half of the alveolar RMS have a t(2;13)(q35;q14) translocation, with about one-fifth having t(1;13)(p36;q14). Other genetic abnormalities have also been identified.112, 113
Treatment is dependent upon tumor size, stage, and influenced by response to first line chemotherapy.
Embryonal rhabdomyosarcoma usually presents as a subcutaneous or deep-seated mass and is the least common of the rhabdomyosarcomas.
On microscopic examination, embryonal rhabdomyosarcoma is a cellular tumor with intermixed myxoid, cystic and hemorrhagic areas. The cell population may vary from small to spindle shaped and cross striations may be seen in the spindle shaped cells.
Immunohistochemical findings are as described for botryoid rhabdomyosarcoma, and are dependent somewhat on the degree of tumor differentiation.
Embryonal rhabdomyosarcomas usually have allelic loss in chromosome region 11p15. Other chromosomal alterations include increased chromosomal numbers specifically related to chromosomes 13, 8, and 2.111, 112 Treatment is dependent upon tumor size, stage, and influenced by response to first line chemotherapy.
OTHER SARCOMAS AND SOFT TISSUE TUMORS
Epithelioid sarcoma has been reported on the vulva, although it is most common on the extremities. Epithelioid sarcoma of the vulva tends to be superficially located and tends to run a more aggressive course than in its usual location in the distal extremities. This may be in part because this tumor, when located in the vulva, often exhibits rhabdoid features, known to be associated with a more aggressive course.114, 115 When rhabdoid features are prominent, it may be difficult to distinguish it from a malignant rhabdoid tumor. Microscopically, the lesion has a granuloma-like appearance, composed of nodules of epithelial-appearing tumor cells with areas of necrosis. The tumor cells resemble nonkeratinizing squamous carcinoma cells; however, they do not connect with the adjacent squamous epithelium and have relatively abundant eosinophilic cytoplasm. The histogenesis of this tumor remains obscure, although it clearly shows features of epithelial differentiation both by electron microscopy and immunohistochemistry. By immunohistochemistry, these tumors are immunoreactive for keratin and epithelial membrane antigen. Partial total deep vulvectomy is the primary treatment of choice and adjuvant therapy, including radiation therapy, may be considered but their value has not been studied in detail and remains unclear.
Malignant rhabdoid tumor is a rare soft tissue malignant neoplasm of uncertain origin that has been reported to present as a labial subcutaneous mass resembling a Bartholin gland abscess in young women of reproductive age.116 These tumors appear as deep subcutaneous, multinodular lesions with poorly defined margins. The polygonal tumor cells resemble rhabdomyocytes, as the name implies; however, the tumor cells are of uncertain origin. Similar to the epithelioid sarcoma, these tumors are immunoreactive for keratins by immunohistochemistry. Unlike epithelioid sarcomas, from which they must be differentiated, the malignant rhabdoid tumor is aggressive and infiltrative, rather than indolent, and extended partial deep vulvectomy, or total vulvectomy, with bilateral inguinal–femoral lymphadenectomy is the treatment of choice; however, adjuvant therapy, including radiation therapy, may be considered but their value has not been studied in detail and remains unclear.
Aggressive angiomyxoma is a soft tissue tumor that is not considered to be malignant in that it has not been reported to metastasize, but is described here because of its relatively aggressive local behavior and because it occurs in the vulva, vagina, and perineum. In the vulva, it usually presents as a subcutaneous mass, most often in the areas of the Bartholin gland. On clinical examination, the mass is typically firm and “rubbery” and may clinically simulate a Bartholin gland cyst. The tumor is very paucicellular and composed of fibroblasts and myofibroblasts within a myxoid stroma and prominent clustered muscular arterioles. Wide local excision is the treatment of choice, and lymphadenectomy is not required. Local recurrence is a significant problem in some cases117 often occurring many years after initial resection (35–72% of cases). These tumors have not been found to metastasize.118, 119
Angiomyofibroblastoma, which is also not a malignant tumor, is included here because of its potential confusion with the aggressive angiomyxoma. Angiomyofibroblastoma, similar to the aggressive angiomyxoma, has a predilection for the vulva and, to a lesser extent, the vagina.120 It typically presents as a polypoid submucosal mass, ranging from 0.5 to 12 cm in size, and grossly appears well circumscribed. Microscopically, the tumor is more cellular than the aggressive angiomyxoma, with areas of hypercellularity alternating with areas of hypocellularity. These tumors are microscopically sharply circumscribed and do not recur after local excision.121 Additional lesions included in the differential diagnosis of aggressive angiomyxoma and angiomyofibroblastoma are fibroepithelial stromal polyps and cellular angiofibroma.
Hemangiopericytoma has been reported in the labia majora and fourchette, presenting as a subcutaneous cystic mass.122 Hemangiopericytomas can be locally aggressive and may metastasize. Patients with larger tumors with high cellularity, mitotic counts of four per 10 HPF or higher and associated tumor necrosis or hemorrhage are considered to be at high risk for recurrence or metastasis.104, Wide local excision is the primary treatment of choice.
Other sarcomas primary in the vulva or directly involving the vulva (a partial list) include angiosarcoma of the vulva;123 lymphangiosarcoma of the thigh after radiation therapy;124 Kaposi sarcoma;125 alveolar soft part sarcoma;126 malignant schwannoma;127 liposarcoma;128, 129, 130 and low grade myofibroblastic sarcoma.131
OTHER PRIMARY TUMORS
Endodermal sinus tumor (yolk sac tumor) is of germ cell origin and primarily occurs in the ovary and testis. It has been rarely reported as a primary tumor of the vulva, vagina, or pelvis primarily in children and young women.132 The tumor presents as a deep subcutaneous mass, but may be polypoid in advanced cases. Microscopic features include Schiller-Duval bodies, but a variety of microscopic patterns have been observed. These tumors produce alpha-fetoprotein, which can be shown by immunohistochemical techniques as well as by elevated serum levels. The treatment of choice is wide, deep local excision with adjuvant chemotherapy. Platinum-based chemotherapy has markedly improved survival rates in patients with this tumor.
Primary vulvar malignant lymphoma is rare. Vulvar malignant lymphoma usually occurs in women of reproductive age as a Bartholin, clitoral, or vulvar mass.133 A variety of lymphoma types have been described, but they are usually of diffuse large B-cell type. Lymphomas must be distinguished from lymphoepithelial carcinoma, which is a rare variant of vulvar squamous carcinoma associated with a prominent chronic inflammatory infiltrate.
Merkel cell tumors are rare but highly aggressive with a high predilection for metastasis. They are of neuroendocrine origin, and in the vulva, they usually present as intradermal nodules. They are often associated with an inflammatory reaction that clinically may mimic an infection or abscess.134, 135 Three histopathologic subtypes are seen: small cell (oat-cell), intermediate, and carcinoid-like (trabecular). The cells are relatively small with hyperchromatic chromatin without nucleoli. Extensive infiltration of the dermis is typical. Cytokeratin stain shows a distinctive perinuclear cytoplastic immunoreactive “dot”. The cells are usually neuron-specific-enolase immunoreactive; however, chromogranin is variable in reactivity and may be negative. The treatment of Merkel cell tumor is wide, deep local excision. If tumor has extended beyond where it is locally resectable or if it has metastasized, chemotherapy protocols as used for small cell carcinoma of the lung may be applicable; however, patients with metastatic Merkel cell tumor have a guarded prognosis.
Peripheral primitive neuroectodermal tumor (pPNET) and Ewing family of tumors are small cell neoplasms which have been reported as rare primary tumors in the vulva. The tumors have been reported predominantly in women of reproductive age, may involve the vulva or vagina, and are usually under 8 cm in greatest dimension.136 The tumor has been reported involving the labia minus or majus and presents as a subcutaneous mass, or polypoid lesion, and may be cyst like or ulcerated.137 Histopathologic features demonstrate a circumscribed tumor with a multilobulated pattern. The tumor has relatively small uniform cells with little cytoplasm, and finely granular hyperchromatic nuclear chromatin. Mitoses are frequent, usually over three per 10 HPF.136 Homer Wright rosettes may be seen, as well as small cystic areas containing with eosinophilic material. On immunohistochemical study the tumor cell cytoplasm has PAS staining material that digests with diastase. The cells have cytoplasmic immunoreactivity for CD99 and may be reactive for vimentin, synaptophysin, and neuron specific enolase (NSE), but are negative for low molecular weight cytokeratin, a feature that distinguishes them from Merkel cell tumor.136, Some cases have the chromosomal translocation t(11;22)(q24;q12), and other chromosomal abnormalities have been described.136 Therapy employed has included partial deep vulvectomy combined with chemotherapy and radiation therapy in some cases. Prognosis is guarded and recurrence and pulmonary metastases may occur within less than a year.135, 136
Other primary tumors of the vulva have been reported, and the reader is referred to comprehensive texts available including those referenced, and continued current publications.4, 26, 41,
METASTATIC TUMORS
Metastatic tumors account for approximately 8% of all vulvar malignancies. Carcinoma of the cervix is the primary tumor in nearly half of the cases reported. Often, the vulva is involved as the tumor spreads distally along the vagina. Less often, metastatic vulvar nodules are noted without vaginal extension and represent hematogenous or lymphatic emboli. Adenocarcinoma of the endometrium or ovary also may spread to the vulva via lymphatic channels, and they are the second and third most common sites of origin of metastasis. Additional metastases from primary tumors of the vagina, bladder, and urethra have been recorded.138, 139 Other less-common metastatic tumors arise from the breast, lung, kidney, stomach, colon, cutaneous melanoma, gestational choriocarcinoma, neuroblastoma, and lymphoma.137, 140, Tumors of the cervix, vagina, urethra, bladder, and rectum may involve the vulva by direct extension.
PROCESSING OF SPECIMENS
Vulvar specimens submitted to a pathology laboratory range from simple punch biopsy of the skin to radical vulvectomy dissections. Proper identification of all tissues with relevant clinical information, exact site of the lesion, and name of the physician is mandatory. When surgical excision is performed on intraepithelial or invasive neoplasms, the surgical margins should be marked with a suture, India ink, or tattoo dye when they are not apparent from gross examination of the specimen. Vulvar biopsy specimens should be specified by site and submitted in separate designated containers. A diagram of the vulva specifying the site of the biopsy specimen is very useful. Discussion with the pathologist is essential in complex cases. The orientation and fixation of the tissue are critical. Small biopsy specimens are first placed on a small piece of absorbent paper or other material to which they will adhere, epidermal side at right angles to the adherent surface. They are then allowed to float (tissue down) on the surface of the fixative (usually 10% buffered formaldehyde solution). This ensures flat fixation and proper orientation so that tangential cutting can be avoided. Larger specimens should be sent to the laboratory in the fresh state, wrapped in moist gauze. When received, such specimens should be promptly laid flat on a corkboard and pinned along the periphery of the tissue. The surgical margins must be identified with India ink or tattoo dye, after which the specimen (tissue down) can be immersed in fixative solution. Specimens of total or partial vulvectomy, with or without inguinal–femoral groin dissection, deserve further attention.
When lymph nodes are submitted, the identification, excision, and proper sampling of all lymph nodes are crucial to the planning of future management. Photography is often useful to document the full extent of tumor in the specimen. Radiographic techniques can be used to assist in the identification of nodes in en bloc specimens. Once all the nodes have been separated from fat, they are sectioned at 2-mm intervals, and alternate-cut surfaces are placed down into a cassette. When lymph nodes are processed in this manner, compared with the usual method of simple bisection, the probability of finding nodal metastasis is enhanced.141
All nodal tissue is submitted for processing with the exception of nodes that grossly contain tumor, which are less extensively sectioned with fewer sections submitted for microscopic examination. After the tissue sections are placed in cassettes, they are submitted by the pathologist to be embedded in paraffin and sectioned for histologic examination.
The correct diagnosis and treatment of vulvar malignancy depend on close cooperation between the clinician and the pathologist as accurate communication is essential and often facilitates patient treatment. Consultation between the pathologist and surgeon provides the flow of information necessary to make an effective decision; the result of such cooperation is an improved quality of patient care.
REFERENCES
Wilkinson EJ, Cox JT, Selim MA, O’Connor DM. Evolution of Terminology for Human-papillomavirus-infection-related vulvar squamous intraepithelial lesions. J Low Genit Tract Dis. 2014 May;19(1):1-7 |
|
Darragh TM, Colgan TJ, Cox JT et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: Background and Consensus Recommendations From the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Lower Genit Tract Dis 16(3): 205-242, 2012 |
|
Crum C, Herrington CS, McCluggage WG, Regauer S, Wilkinson EJ Tumors of the Vulva. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH, eds.WHO Classification of Tumours of Female Reproductive Organs. Lyons:IARC;2014 |
|
Heller DS. Report of a new ISSVD classification of VIN. J Lower Genit Tract Dis 11(1): 46-47, 2007 |
|
Sideri M, Jones RW, Wilkinson EJ, Preti M, Heller DS, Scurry J, Haefner H, Neill S. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. J Reprod Med 50(11): 807–10, 2005 |
|
Wilkinson EJ, Friedrich EG Jr, Fu YS: Multicentric nature of vulvar carcinoma in situ. Obstet Gynecol 58: 59, 1981 |
|
Friedrich EG Jr, Wilkinson EJ, Fu YS: Carcinoma in situ of the vulva: A continuing challenge. Am J Obstet Gynecol 136: 830, 1980 |
|
McNally OM, Mulvany NJ, Pagano R, Quinn MA, Rome RM. VIN 3: a clinicopathologic review. Int J Gynecol Cancer 2002;12:490-495. |
|
Van de Nieuwenhof HP, van der Avoort IAM, de Hullu JA. Review of squamous premalignant vulvar lesions. Crit Rev Onc Hem 2008;68:131-156. |
|
Chafe W, Richards A, Morgan LS et al: Unrecognized invasive carcinoma in vulvar intraepithelial neoplasia (VIN). Gynecol Oncol 31; 154, 1988 |
|
Jones RW, McLean MR: Carcinoma in situ of the vulva: A review of 31 treated and 5 untreated cases. Obstet Gynecol 68: 499, 1986 |
|
Friedrich EG: Reversible vulvar atypia. Obstet Gynecol 39: 173, 1972 |
|
Hart WR: Vulvar intraepithelial neoplasia: Historical aspects and current status. Int J Gynecol Pathol 20: 16, 2001 |
|
Jones R, Rowan D, Stewart A. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome of 405 women. Obstet Gynecol 2005;106:1319-1326 |
|
Stephenson RD and Denehy TR. Rapid spontaneous regression of acute-onset vulvar intraepithelial neoplasia 3 in young women: a case series. J Lower Genit Tract Dis 16(1): 56-58, 2012 |
|
Toki T, Kurman RJ, Park JS et al: Probable nonpapillomavirus etiology of squamous cell carcinoma of the vulva in older women: A clinicopathologic study using in situ hybridization and polymerase chain reaction. Int J Gynecol Pathol 10: 107, 1991 |
|
Yang B, Hart WR: Vulvar Intraepithelial Neoplasia of the Simplex (Differentiated) type. A clinicopathologic study including analysis of HPV and p53 expression. Am J Surg Pathol 24: 429–441, 2000 |
|
Kruse AJ, Bottenberg MJ, Tosserams J, Slangen B, van Marion AM, van Trappen PO. The absence of high-risk HPV combined with specific p53 and p16INK4a expression patterns points to the HPV-independent pathway as the causative agent for vulvar squamous cell carcinoma and its precursor simplex VIN in a young patient. Int J Gynecol Pathol 27(4): 591–5, 2008 |
|
Srodon M, Stoler MH, Baber GB, Kurman RJ. The Distribution of Low and High Risk HPV Types in Vulvar and Vaginal Intraepithelial Neoplasia (VIN and VaIN). Am J Surg Pathol 30(12): 1513-4, 2006 |
|
McCluggage WG. Recent developments in vulvovaginal pathology. Histopath 2009;54:156-73. |
|
Wilkinson EJ, Stone IK: Atlas of Vulvar Disease, 2nd edn.Philadelphia, Wolters Kluwer/Lippincott-Williams and Wilkins, 2008 |
|
Shatz P, Bergeron C, Wilkinson EJ et al: Vulvar intraepithelial neoplasia and skin appendage involvement. Obstet Gynecol 74: 769, 1989 |
|
Zaino RJ: Carcinoma of the vulva, urethra and Bartholin's gland. In Wilkinson EJ (ed): Contemporary Issues in Surgical Pathology: Pathology of the Vulva and Vagina, pp 119–153, Vol 9. New York, Churchill Livingstone, 1987 |
|
Hillemanns P, Untch M, Dannecker C et al: Photodynamic therapy of vulvar intraepithelial neoplasia using 5-aminolevulinic acid. Int J Cancer 85: 659, 2000 |
|
Davis G, Wentworth J, Richard J: Self-administered topical Imiquimod treatment of vulvar intraepithelial neoplasia: A report of four cases. J Reprod Med 45: 619, 2000 |
|
Todd RW, Etherington IJ, Luesley DM. The effects of 5% imiquimod cream on high-grade vulval intraepithelial neoplasia. Gynecol Oncol. 2002 Apr;85(1):67-70. |
|
Wilkinson, EJ: “Premalignant and Malignant Tumors of the Vulva.”, In: Blaustein’s Pathology of the Female Genital Tract, 6th ed., Kurman, R.J., (ed.), Springer-Verlag, New York, pp. 55–103, 2011. |
|
Wilkinson EJ, Brown HM: Pagetoid urothelial intraepithelial neoplasia (PUIN): Differentiation from Paget disease of cutaneous or ano-rectal origin. Mod Pathol 14: 147A, 2001 |
|
Fishman DA, Chambers SK, Schwartz PE et al: Extramammary Paget's disease of the vulva. Gynecol Oncol 56: 266, 1995 |
|
Ewing TL: Paget's disease of the vulva treated by combined surgery and laser. Gynecol Oncol 43: 137, 1991 |
|
Ridley CM: The Vulva, New York, Churchill Livingstone, 1988 |
|
Hatch KD, Davis JR. Complete resolution of Paget disease of the vulva with imiquimod cream. J Low Genit Tract Dis 12(2): 90–4, 2008 |
|
Judson LJ, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol 2006; 107:1018-1022. |
|
Al-Ghamdi A, Freedman D, Miller D, Poh C, Rosin M, Zhang L, Gilks CB. Vulvar squamous cell carcinoma in young women: a clinicopathologic study of 21 cases. Gynecol Oncol. 2002 Jan;84(1):94-101. |
|
Hording U, Daugaard S, Junge J et al: Human papillomaviruses and multifocal genital neoplasia. Int J Gynecol Pathol 15: 230, 1996 |
|
Rodke G, Friedrich EG Jr, Wilkinson EJ: Malignant potential of mixed vulvar dystrophy (lichen sclerosus associated with squamous cell hyperplasia). J Reprod Med 33: 545, 1988 |
|
Hacker NF, Van der Velden J: Conservative management of early vulvar cancer. Cancer 71 (Suppl 4): 1673, 1993 |
|
Wilkinson EJ: Protocol for the examination of specimens from patients with carcinomas and malignant melanomas of the vulva. Arch Pathol Lab Med 124: 51, 2000 |
|
Creasman WT: Editorial: New gynecologic cancer staging. Gynecol Oncol 58: 157, 1995 |
|
Kurman RJ, Toki T, Schiffman MH. Basaloid and warty carcinomas of the vulva. Distinctive types of squamous cell carcinoma frequently associated with human papillomaviruses. Am J Surg Pathol 1993;17: 133-145. |
|
Toki T, Kurman RJ, Park JS, et al. Probable nonpapillomavirus etiology of squamous cell carcinoma of the vulva in older women: a clinicopathologic study using in situ hybridization. Int J Gynecol Pathol 1991;10: 107-125. |
|
Kurman RJ, Ronnett B, Wilkinson E. Tumors of The Cervix, Vagina and Vulva. American Registry of Pathology. 4th series #13, 2010 |
|
Wilkinson EJ, Croker BP, Friedrich EG Jr et al: Two distinct pathologic types of giant cell tumor of the vulva: A report of two cases. J Reprod Med 33: 519, 1988 |
|
Santeusanio G, Schiaroli S, Anemona L, et al (1991) Carcinoma of the vulva with sarcomatoid features: a case report with immunohistochemical study. Gynecol Oncol |
|
Lasser A, Cornog JL, Morris JM: Adenoid squamous cell carcinoma of the vulva. Cancer 33: 224, 1974 |
|
Lomo L and Crum CP. Papillary carcinoma of the vulva. Mod Pathol 204A:17, 2004. |
|
Carr KA, Bulengo S, Weiss LM, et al (1992) Lymphoepithelioma-like carcinoma of the skin. Am J Surg Pathol 16:909–913. |
|
Tran TA, Carlson JA. Plasmacytoid carcimona of the Vulva Int J Gynecol Pathol. 27(4):601-5,2008 |
|
Kneale BL: Microinvasive cancer of the vulva: Report of the International Society for the Study of Vulvar Disease Task Force, VIIth Congress. J Reprod Med 29: 454, 1984 |
|
Wilkinson EJ, Rico JM, Pierson KK: Microinvasive carcinoma of the vulva. Int J Gynecol Pathol 1: 29, 1982 |
|
Yoder BJ, Rufforny I, Massoll N, Wilkinson E, Stage IA Vulvar Squamous Cell Carcinoma; An Analysis of Tumor Invasive Characteristics and Risk. Amer J Surg Pathol. 2008 May;32(5):765-72 |
|
Wilkinson EJ: Superficially invasive carcinoma of the vulva. In Wilkinson EJ (ed): Contemporary Issues in Surgical Pathology: Pathology of the Vulva and Vagina, p 103, Vol 9. New York, Churchill Livingstone, 1987 |
|
Underwood JW, Adcock LL, Okagahi T: Adenosquamous carcinoma of skin appendages (adenoid squamous cell carcinoma, pseudoglandular squamous cell carcinoma, adenoacanthoma of sweat gland of Lever) of the vulva. A clinical and ultrastructural study. Cancer 42: 1851, 1978 |
|
Jones MA, Mann EW, Caldwell CL et al: Small cell neuroendocrine carcinoma of Bartholin's gland. Am J Clin Pathol 94: 439, 1990 |
|
Husseinzadeh N, Wesseler T, Newman N et al: Neuroendocrine (Merkel cell) carcinoma of the vulva. Gynecol Oncol 29: 105, 1988 |
|
Gil-Moreno A, Garcia-Jimenez A, Gonzalez-Bosquet J et al: Merkel cell carcinoma of the vulva. Gynecol Oncol 64: 526, 1997 |
|
Chen KTK: Merkel's cell (neuroendocrine) carcinoma of the vulva. Cancer 73: 2186, 1994 |
|
Tiltman AJ, Knutzen VK: Primary adenocarcinoma of the vulva originating in misplaced cloacal tissue. Obstet Gynecol 51: 330, 1978 |
|
DeLasCasas LE, Kurtycz DF, Caya JG: Inguinal lymph node dissections in patients with vulvar carcinoma. How many lymph nodes is enough? Mod Pathol 14: 135A, 2001 |
|
Burke TW, Eifel PJ, McGuire WP et al: Vulva. In Principles and Practice of Gynecologic Oncology, pp 775–810, 3rd ed. Philadelphia, Lippincott Williams Wilkins, 2000 |
|
Brisigotti M, Moreno A, Murcia C et al: Verrucous carcinoma of the vulva. A clinicopathologic and immunohistochemical study of five cases. Int J Gynecol Pathol 8: 1, 1989 |
|
Kondi-Paphitis A, Deligeorgi-Politi H, Liapis A et al: Human papilloma virus in verrucous carcinoma of the vulva: An immunopathological study of three cases. Eur J Gynaecol Oncol 19: 319, 1998 |
|
Andersen ES, Sorensen IM: Verrucous carcinoma of the female genital tract: Report of a case and review of the literature. Gynecol Oncol 30: 427, 1988 |
|
Mehta RK, Rytima E, Sterling JC: Treatment of verrucous carcinoma of vulva with acitretin. Br J Dermatol 142: 1195, 2000 |
|
Feakins RM, Lowe DG: Basal cell carcinoma of the vulva: A clinicopathologic study of 45 cases. Int J Gynecol Pathol 16: 319, 1997 |
|
Takahashi H. Non-ulcerative basal cell carcinoma arising on the genitalia.J Dermatol 2000; 27(12):798-801. |
|
Mulayim N, Foster Silver D, Tolgay Ocal I, Babalola E. Vulvar basal cell carcinoma: two unusual presentations and review of the literature. Gynecol Oncol 2002; 85(3):532-537. |
|
Gorlin RJ: Nevoid basal cell carcinoma syndrome. Derm Clin No Am 13: 113, 1995 |
|
Nehal KS, Levine VJ, Ashinoff R: Basal cell carcinoma of the genitalia. Dermatol Surg Dec 24: 1361, 1998 |
|
Morita Y, Hikage S, Oginom: Adenoid cystic carcinoma of Bartholin's gland. Int J Gynaecol Obstet Sep; 54: 279, 1996 |
|
Tellechea O, Reis JP, Domingues JC, Baptista AP. Monoclonal antibody Ber EP4 distinguishes basal-cell carcinoma from squamous-cell carcinoma of the skin. Am J Dermatopathol. 1993;15(5):452-53 |
|
Benedet JL, Miller DM, Ehlen TG: Basal cell carcinoma of the vulva: clinical features and treatment results in 28 patients. Obstet Gynecol 90: 765, 1997 |
|
Perrone T, Twiggs LB, Adcock LL et al: vulvar basal cell carcinoma. An infrequently metastasizing neoplasm. Int J Gynecol Pathol 6: 152, 1987 |
|
Piura B, Rabinovich A, Dgani R: Basal cell carcinoma of the vulva. J Surg Oncol Mar; 70: 172, 1999 |
|
Ragnarsson-Olding BK, Kanter-Lewensohn LR, Lagerlof B et al: Malignant melanoma of the vulva in a nationwide, 25-year study of 219 Swedish females. Cancer 86: 1273, 1999 |
|
Dunton CJ, Kautzky M, Hanau C. Malignant melanoma of the vulva: a review. Obstet Gynecol Surv. 1995 Oct;50(10):739-46. |
|
Podratz KC, Gaffey TA, Symmonds RE et al: Melanoma of the vulva: An update. Gynecol Oncol 16: 153, 1983 |
|
Tasseron EW, van der Esch EP, Hart AA et al: A clinicopathological study of 30 melanomas of the vulva. Gynecol Oncol 46: 170, 1992 |
|
Benda JA, Platz CE, Anderson B: Malignant melanoma of the vulva: A clinical-pathologic review of 16 cases. Int J Gynecol Pathol 5: 202, 1986 |
|
Clark WH, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behaviour of primary human malignant melanoma of the skin. Cancer Res 1969;29:705-26. |
|
Chung AF, Woodruff JM, Lewis JL: Malignant melanoma of the vulva. Obstet Gynecol 45: 638, 1975 |
|
Breslow A: Tumor thickness, level of invasion and node dissection in stage I cutaneous melanoma. Ann Surg 182; 572, 1975 |
|
Raspagliesi F, Ditto A, Paladini D et al: Prognostic indicators in melanoma of the vulva. Ann Surg Oncol Dec; 7: 738, 2000 |
|
Ragnarsson-Olding BK, Nilsson BR, Kanter-Lewensohn LR et al: Malignant melanoma of the vulva in a nationwide, 25-year study of 219 Swedish females-predictors of survival. Cancer 86: 1285, 1999 |
|
Beller U, Demopoulos RI, Beckman EM: Vulvovaginal melanoma: A clinicopathologic study. J Reprod Med 31: 315, 1986 |
|
Ronan SG, Eng AM, Briele HA et al: Malignant melanoma of the female genitalia. J Am Acad Dermatol 22: 428, 1990 |
|
Trimble EL: Melanomas of the vulva and vagina. Oncology 10: 1017, 1996 |
|
Trimble EL, Lewis JL, Jr, Williams LL et al: Management of vulvar melanoma. Gynecol Oncol 45: 254, 1992 |
|
Woolcott RJ, Henry RJ, Houghton CR: Malignant melanoma of the vulva. J Reprod Med 33: 699, 1988 |
|
Verschraegen CF, Benjapibal M, Supakarapongkul W, Levy LB, Ross M, Atkinson EN, Bodurka-Bevers D, Kavanagh JJ, Kudelka AP, Legha SS. Vulvar melanoma at the M. D. Anderson Cancer Center: 25 years later. Int J Gynecol Cancer. 2001 Sep-Oct;11(5):359-64. |
|
Maffioli L, Sturm E, Roselli M et al: State of the art of sentinel node biopsy in oncology. Tumori 86: 263, 2000 |
|
Abramova L, Parekh J, Irvin WP Jr, Rice LW, Taylor PT Jr, Anderson WA, Slingluff CL Jr. Sentinel node biopsy in vulvar and vaginal melanoma: presentation of six cases and a literature review. Ann Surg Oncol. 2002 Nov;9(9):840-6. |
|
Johnson TC, Kumar NB, White CD et al: Prognostic features of vulvar melanoma: A clinicopathologic analysis. Int J Gynecol Pathol 5: 110, 1986 |
|
Rock B: Pigmented lesions of the vulva. Dermatol Clin 10: 361, 1992 |
|
Blickstein I, Feldberg E, Dgani R et al: Dysplastic vulvar nevi. Obstet Gynecol 78: 968, 1991 |
|
Banerjee SS, Harris M: Morphologic and immunophenotypic variations in malignant melanoma. Histopathology May; 36: 387, 2000 |
|
Cardosi RJ, Speights A, Fiorica JV, Grendys EC Jr, Hakam A, Hoffman MS. Bartholin's gland carcinoma: a 15-year experience. Gynecol Oncol. 2001 Aug;82(2):247-51 |
|
Jones MA, Mann EW, Caldwell CL, Tarraza HM, Dickersin GR, Young RH. Small cell neuroendocrine carcinoma of Bartholin's gland. Am J Clin Pathol. 1990 Oct;94(4):439-42. |
|
Obermair A, Koller S, Crandon AJ: Primary Bartholin's gland carcinoma: A report of seven cases. Aust NZ J Obstet Gynaecol 41: 78, 2001 |
|
DiBonito L, Patriarca S, Falconieri G: Aggressive “breast-like” adenocarcinoma of vulva. Pathol Res Pract 188: 211, 1992 |
|
Wick MR, Goellner JR, Wolfe IT III et al: Vulvar sweat gland carcinoma. Arch Pathol Lab Med 109: 43, 1985 |
|
Abbott JJ, Ahmed I. Adenocarcinoma of mammary-like glands of the vulva: Report of a case and review of the literature. Am J Dermatopathol. 2006 Apr;28(2):127-33. Review |
|
Nielsen GP, Rosenberg AE, Koerner FC et al. Smooth muscle tumors of the vulva. A clinicopathologic study of 25 cases and review of the literature. Am J Surg Path 1996; 20: 779-93 |
|
Taylor RN, Bottles K, Miller TR, Braga CA. Malignant fibrous histiocytoma of the vulva. Obstet Gynecol 1985:66; 145-8 |
|
Schwartz BM, Kuo DYS, Goldberg GL. Dermatofibrosarcoma protuberans of the vulva: a rare tumor presenting during pregnancy in a teenager. J Low Genit Tract Dis 1999;3: 139-42 |
|
LiVolsi VA, Brooks JJ: Soft tissue tumors of the vulva. In Wilkinson EJ (ed): Contemporary Issues in Surgical Pathology: Pathology of the Vulva and Vagina, pp 209–238, Vol 9. New York, Churchill Livingstone, 1987 |
|
Nucci MR, Fletcher CDM: Vulvovaginal soft tissue tumors: Update and review. Histopathology 36: 97, 2000 |
|
Martelli H, Oberlin O, Rey A et al. Conservative treatment for girls with nonmetastatic rhabdomyosarcoma of the genital tract: A report form the study committee of The International Society of Pediatric Oncology. J Clin Oncol 1999; 17: 2117-22 |
|
Weiss SW, Goldblum JR. Rhabdomyosarcoma. In Weiss SW, Goldblum JR, Einzinger FM eds. Einzinger and Weiss's Soft Tissue Tumors, 4th ed. St Louis: Mosby; 2001: 785-835 |
|
Copeland LJ, Gershenson DM, Saul PB et al. Sarcoma botryoides of the female genital tract. Obstet Gynecol 1985; 66: 262-6 |
|
Raney RB, Crist W, Hays D et al. Soft tissue sarcoma of the perineal region childhood. A report from the Intergroup Rhabdomyosarcoma Studies I and II, 1972 through 1984. Cancer 1990; 65: 2787-92 |
|
McDowell HP. Update on childhood rhabdomyosarcoma. Arch Dis Child 2003; 88:354-7 |
|
Parham DM, Barr FG. Alveolar Rhabdomyosarcoma. In: Fletcher CD, Unni KK, Mertens F eds. World Health Organization classification of tumors, pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press, 2002:150-2 |
|
Weissmann D, Amenta PS, Kantor GR: Vulvar epithelioid sarcoma metastatic to the scalp. A case report and review of the literature. Am J Dermatopathol 12: 462, 1990 |
|
Argenta PA, Thomas S, Chura JC. Proximal-type epithelioid sarcoma vs. malignant rhabdoid tumor of the vulva: a case report, review of the literature, and an argument for consolidation. Gynecol Oncol. 2007 Oct;107(1):130-5. Epub 2007 Aug 9. Review. |
|
Igarashi T, Sasano H, Konno R, et al. Malignant rhabdoid tumor of the vulva: case report with cytological, immunohistochemical, ultrastructural and DNA ploidy studies and a review of the literature. Pathol Int 1998;48: 887 |
|
Fetsch JF, Laskin WB, Lefkowitz M et al: Aggressive angiomyxoma. A clinicopathologic study of 29 female patients. Cancer 78: 79, 1996 |
|
Begin LR, Clement PB, Kirk ME et al: Aggressive angiomyxoma of pelvic soft parts: A clinicopathologic study of nine cases. Hum Pathol 16: 621, 1985 |
|
Steeper T, Rosal J: Aggressive angiomyxoma of the female pelvis and perineum: Report of nine cases. Am J Surg Pathol 7: 463, 1983 |
|
Laskin WB, Fetsch JF, Tavassoli FA: Angiomyofibroblastoma of the female genital tract: Analysis of 17 cases including a lipomatous variant. Hum Pathol 28: 1046, 1997 |
|
Ockner DM, Sayadi H, Swanson PE et al: Genital angiomyofibroblastoma. Comparison with aggressive angiomyxoma and other myxoid neoplasms of skin and soft tissue. Am J Clin Pathol 107: 36, 1997 |
|
ZaKut H, Lotan M, Lipnilsky M: Vulvar hemangiopericytoma: A case report and review of previous cases. Acta Obstet Gynecol Scand 64: 619, 1985 |
|
Nirenberg A, Ostor AG, Slavin J et al: Primary vulvar sarcomas. Int J Gynecol Pathol 14: 55, 1995 |
|
Huey GR, Stehman FB, Roth LM et al: Lymphangiosarcoma of the edematous thigh after radiation therapy for carcinoma of the vulva. Gynecol Oncol 20: 394, 1985 |
|
Agarossi A, Vago L, Lazzarin A et al: Letter: Vulvar Kaposi's sarcoma: A case report. Ann Oncol 2: 609, 1991 |
|
Shen IT, D'Ablaing G, Morrow CP: Alveolar soft part sarcoma of the vulva: Report of first case and review of literature. Gynecol Oncol 13: 120, 1982 |
|
Terada KY, Schmidt TW, Roberts JA: Malignant schwannoma of the vulva: A case report. J Reprod Med 33: 969, 1988 |
|
Brooks JJ, LiVolsi VA: Liposarcoma presenting on the vulva. Am J Obstet Gynecol 156: 73, 1987 |
|
Genton DY, Maroni ES: Vulval liposarcoma. Arch Gynecol 240: 63, 1987 |
|
Nucci MR, Fletcher CD: Liposarcoma (atypical lipomatous tumors) of the vulva: A clinicopathologic study of six cases. Int J Gynecol Pathol 17: 17, 1998 |
|
Roth TM, Fratkin J, Woodring TC, McGehee RP. Low-grade myofibroblastic sarcoma of the vulva. Gynecol Oncol. 2004 Jan;92(1):361-4. |
|
Flanagan CW, Parker JR, Mannel RS et al: Primary endodermal sinus tumor of the vulva: A case report and review of the literature. Gynecol Oncol 66: 515, 1997 |
|
Tuder RM: Vulvar destruction by malignant lymphoma. Gynecol Oncol 45: 52, 1992 |
|
Husseinzadeh N, Whesseler T, Newman N et al: Neuroendocrine (Merkel cell) carcinoma of the vulva. Gynecol Oncol 29: 105, 1988 |
|
Cliby W, Soisson AP, Berchuck A et al: Stage I small cell carcinoma of the vulva treated with vulvectomy, lymphadenectomy, and adjuvant chemotherapy. Cancer 67: 2415, 1991 |
|
McCluggage WG, Sumathi VP, Nucci MR, Hirsch M, Dal Cin P, Wells M, Flanagan AM, Fisher C. Ewing family of tumours involving the vulva and vagina: report of a series of four cases. J Clin Pathol. 2007 Jun;60(6):674-80. Review. |
|
Vang R, Taubenberger JK, Mannion CM, et al. Primary vulvar and vaginal extraosseous Ewing's sarcoma/peripheral neuroectodermal tumor: diagnostic confirmation with CD99 immunostaining and reverse transcriptase-polymerase chain reaction. Int J Gynecol Pathol. 2000 Apr;19(2):103-9. |
|
Mazur MT, Hsueh S, Gersell DJ: Metastases to the female genital tract: Analysis of 325 cases. Cancer 53: 1978, 1984 |
|
Powell CS, Jones PA: Carcinoma of the bladder with a metastasis in the clitoris. Br J Obstet Gynecol 90: 380, 1983 |
|
Radman HM: Metastatic melanoma of the vulva. MD State Med J 30: 60, 1981 |
|
Wilkinson EJ, Hause LL, Hoffman RG et al: Occult axillary lymph node metastases in invasive breast carcinoma: Characteristics of the primary tumor and significance of the metastases. Pathol Annu 17: 67, 1982 |